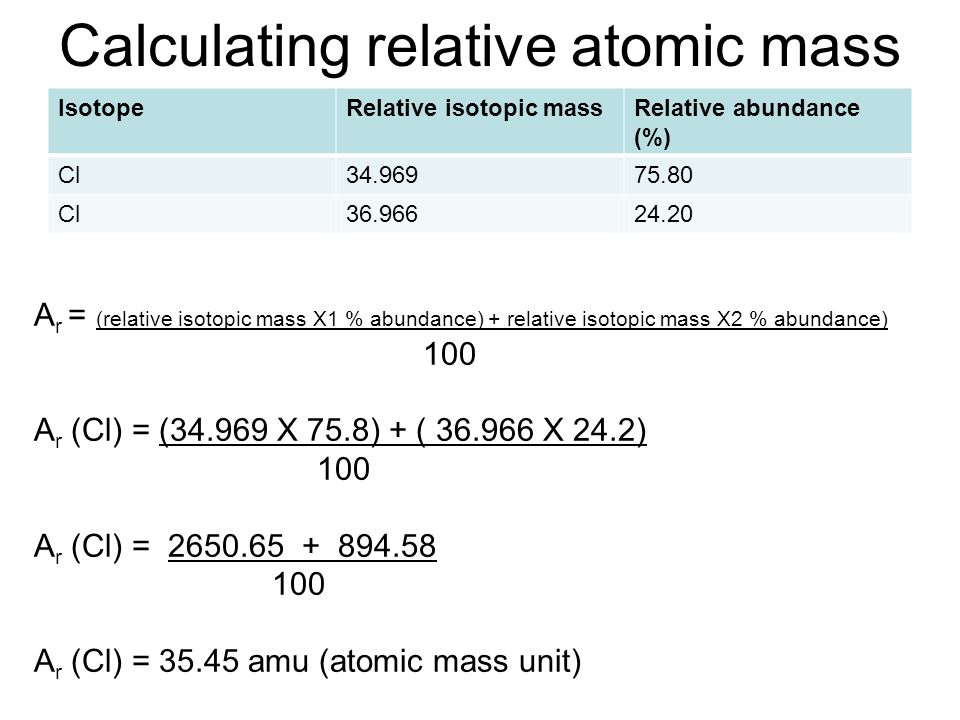
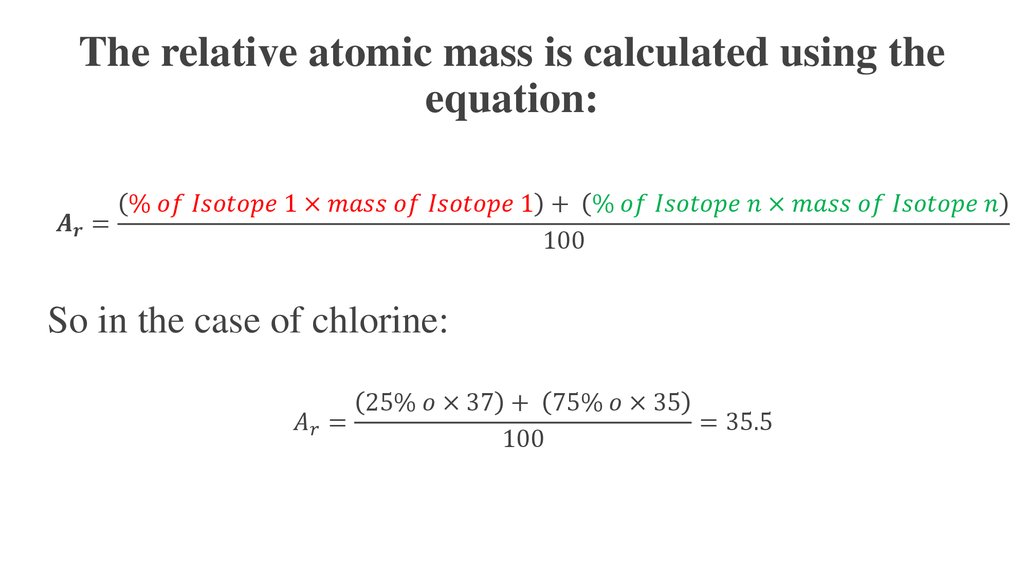
How To Work Out The Relative Atomic Mass Of Chlorine In order to calculate the relative atomic mass A r of chlorine the following steps are used Multiply the mass of each isotope by its relative abundance Add those together Divide by the sum of the relative abundances normally 100
Dec 12 2024 nbsp 0183 32 To calculate A r multiply each isotope s mass by its abundance then add the results using A r Isotopic mass 215 Abundance Elements like fluorine have a fixed A r while isotopic variation causes elements like chlorine In any sample of chlorine 75 per cent of the atoms are 35 Cl and the remaining 25 per cent are 37 Cl The relative atomic mass is worked out using the following formula illustrated for two
How To Work Out The Relative Atomic Mass Of Chlorine

How To Work Out The Relative Atomic Mass Of Chlorine
https://i.ytimg.com/vi/IyiLONYZzmc/maxresdefault.jpg

Calculate The Atomic Mass average Of Chlorine Using The Following
https://i.ytimg.com/vi/nCMtan7zvsc/maxresdefault.jpg

Atomic Mass Atomic Number Valency Of First 30 Elements YouTube
https://i.ytimg.com/vi/wf2saEMQ5Zc/maxresdefault.jpg
It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element It also looks at the problems thrown up by elements with Example 2 Chlorine with relative atomic mass of 35 5 contains two isotopes 35 Cl and 37 Cl what is the relative abundance of each isotope in a sample of chlorine Solution 35 5 A 100
In these lessons we will learn about Isotopes Isotope Notation Atomic Mass Unit amu and how to calculate the Atomic Mass of an element The following diagrams show the isotopes of chlorine and how to calculate the relative May 25 2022 nbsp 0183 32 e g calculate the relative atomic mass for chlorine given that it has 2 isotopes 35 Cl with an abundance of 75 and 37 Cl with an abundance of 25 A r 35 x 75 37 x 25 100 35 5 We can also use the mass
More picture related to How To Work Out The Relative Atomic Mass Of Chlorine

How To Find The Mass Of One Atom Of Chlorine Cl YouTube
https://i.ytimg.com/vi/JfyzPWf7Wcg/maxresdefault.jpg

EL Calculation Of Relative Atomic Mass From Mass Spectrometry Data
https://i.ytimg.com/vi/3eNrvay8LNY/maxresdefault.jpg

Relative Atomic Mass And Atomic Mass Unit amu Fundamental Of
https://i.ytimg.com/vi/lFkYI7U0YF4/maxresdefault.jpg
To work out relative atomic mass multiply the mass of each isotope by its relative abundance Then add those numbers together and divide by the sum of the relative abundances The calculation for working out the relative atomic mass Nov 15 2024 nbsp 0183 32 To find the relative atomic mass of chlorine we ll follow these steps Identify the mass number and natural abundance of each isotope Multiply the mass number of each isotope by its percentage abundance This step
The relative atomic mass A r is calculated from 2 things mass numbers of its isotopes and abundance of these isotopes Let s use Chlorine as an example Chlorine naturally exists as Calculate the relative atomic mass of chlorine Predict and sketch the mass spectra for chlorine molecules A sample contain the element Zinc has a relative atomic mass of 64 66 The
Calculating relative atomic mass Dynamic Periodic Table Of Elements
https://periodictable.me/wp-content/uploads/2018/08/Calculatingrelativeatomicmass.jpg
Atomic Mass Online Presentation
https://cf.ppt-online.org/files/slide/l/LFEcGVbymhnABN2IwtpovuYiOHjW478DRU9T3s/slide-6.jpg
How To Work Out The Relative Atomic Mass Of Chlorine - Relative atomic mass is defined and explained below and examples of how to calculate it from data The relative atomic mass scale is now based on an isotope of carbon namely carbon