How To Find The Relative Atomic Mass Simple the definition lies in the meaning itself The mass of an atom is refereed as its atomic mass Measured in amu or atomic mass unit Commonly the atomic mass is calculated by adding the number of protons and neutrons together whereas electrons are ignored Expressed in grams or any other units to measure weight
Jul 10 2017 nbsp 0183 32 What is the relative atomic mass of thallium We have two different masses and a known percentage of each 0 295 x 203 0 705 x 205 59 885 144 525 204 4 This is what we would expect a slight shift to the higher mass from the 50 50 average of 204 because of the larger percentage of Tl 205 in the sample May 22 2018 nbsp 0183 32 Relative atomic mass quot Mass of an atom of element quot 1 12 quot Mass of an atom of quot C 12 uarrThe above equation is a formula to find relative atomic mass of an element
How To Find The Relative Atomic Mass

How To Find The Relative Atomic Mass
https://m.media-amazon.com/images/I/418sPYJ2CHL._SL500_.jpg

Relative Isotopic Mass YouTube
https://i.ytimg.com/vi/ID4RSzgg0Nk/maxresdefault.jpg

Relative Formula Mass YouTube
https://i.ytimg.com/vi/u7Sm6yk2g0M/maxresdefault.jpg
Dec 30 2017 nbsp 0183 32 Refer to the explanation Relative formula mass quot M quot r is determined by multiplying the subscript of each element in the formula by its relative atomic mass quot A quot r on the periodic table The results for each element are added together to calculate the relative formula mass The unified mass unit or dalton u or Da is applied to the relative formula and atomic Oct 4 2016 nbsp 0183 32 Find out the mean of all an atom s isotopic masses An atom s isotopic mass is the sum of its protons and neutrons we ll exclude electrons because they ve negligible weight Then we work out the mean of its isotopes using their abundances For example chlorine has two isotopes quot quot 35 quot Cl quot at about 75 abundance and quot quot 37 quot Cl quot at about 25 abundance
Jan 3 2014 nbsp 0183 32 The term quot atomic mass quot refers to the mass of a single atom The mass of a single atom of carbon 12 is defined as exactly 12 u The term atomic mass is also often used though technically incorrectly to refer to the average atomic mass of all of the isotopes of an element This second definition is actually the relative atomic mass of an element a single average Dec 13 2015 nbsp 0183 32 Simply put an element s naturally occurring isotopes will contribute to the average atomic mass of the element proportionally to their abundance color blue quot avg atomic mass quot sum i quot isotope quot i xx quot abundance quot x When it comes to the actual calculation it s easier to use decimal abundances which are simply percent abundances divided by
More picture related to How To Find The Relative Atomic Mass

Relative Atomic Mass Abundance A level Chemistry YouTube
https://i.ytimg.com/vi/H_a8PGRJJEA/maxresdefault.jpg

EASY WAY To Calculate RELATIVE ATOMIC MASS GCSE Chemistry YouTube
https://i.ytimg.com/vi/TrUU2Sw9pUQ/maxresdefault.jpg

Relative Formula Mass GCSE Science GCSE Chemistry Get To Know
https://i.ytimg.com/vi/xpXOaYDiXO8/maxresdefault.jpg
Oct 22 2015 nbsp 0183 32 In your case magnesium 24 will have an atomic mass of quot 24 u quot because it contains 12 protons and 12 neutrons Here u represents the unified atomic mass unit and is equal to the mass of one nucleon proton or neutron Likewise magnesium 25 will have an atomic mass of quot 25 u quot because it contains 12 protons aqnd 13 neutrons Aug 14 2016 nbsp 0183 32 Neon 20 has a mass of 19 9924 amu Neon 21 has a mass of 20 9940 amu Neon 22 has a mass of 21 9914 amu The relative abundance of 20Ne is 90 92 and 21 Ne is 257 and 22 Ne is 8 82
[desc-10] [desc-11]

S1 2 2 Calculating Relative Atomic Mass YouTube
https://i.ytimg.com/vi/DjJqgx0HzyY/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYACzgWKAgwIABABGH8gEygUMA8=&rs=AOn4CLDnmI1WSowCC55lOda-IAl5ExzMdA
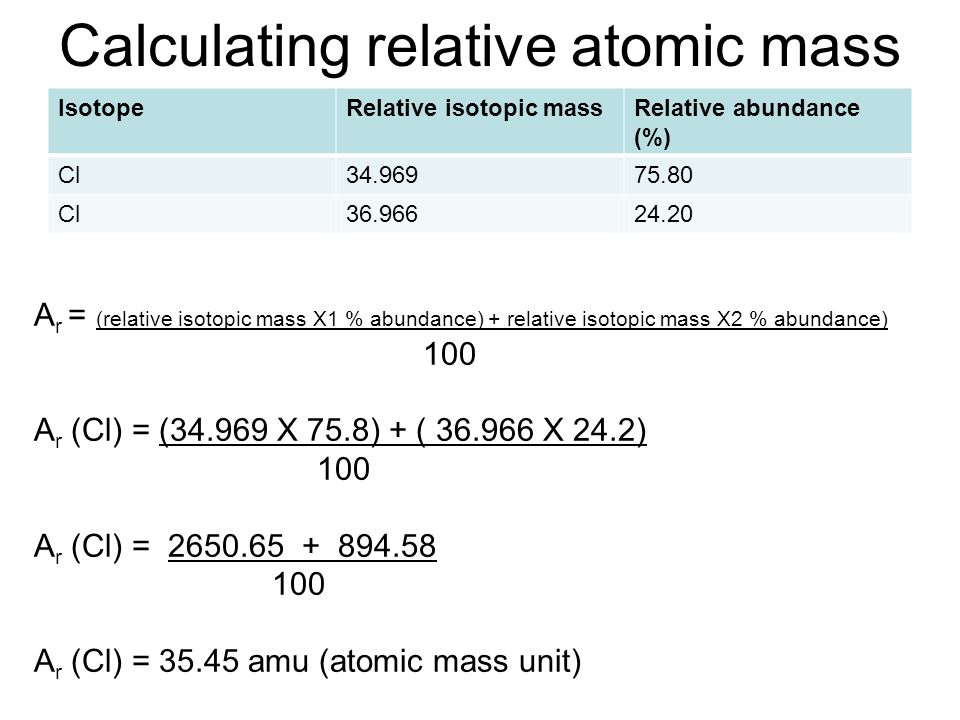
Calculating relative atomic mass Dynamic Periodic Table Of Elements
https://periodictable.me/wp-content/uploads/2018/08/Calculatingrelativeatomicmass.jpg
How To Find The Relative Atomic Mass - [desc-12]