How To Balance Redox Reaction By Oxidation Number Method May 1 2025 nbsp 0183 32 One way to balance redox reactions is by keeping track of the electron transfer using the oxidation numbers of each of the atoms For the oxidation number change method
Learn how to balance redox reactions using the Oxidation Number Change Method and the Ion Electron Method Nov 7 2021 nbsp 0183 32 Redox reaction can be balanced by either of the following two methods Balance the following equations by the oxidation number method or ion electron method The oxidation number of an oxidizing agent decreases and
How To Balance Redox Reaction By Oxidation Number Method

How To Balance Redox Reaction By Oxidation Number Method
https://i.ytimg.com/vi/dF5lB7gRtcA/maxresdefault.jpg

Balancing Redox Reactions By Half Reaction Method YouTube
https://i.ytimg.com/vi/G_igHCS066M/maxresdefault.jpg
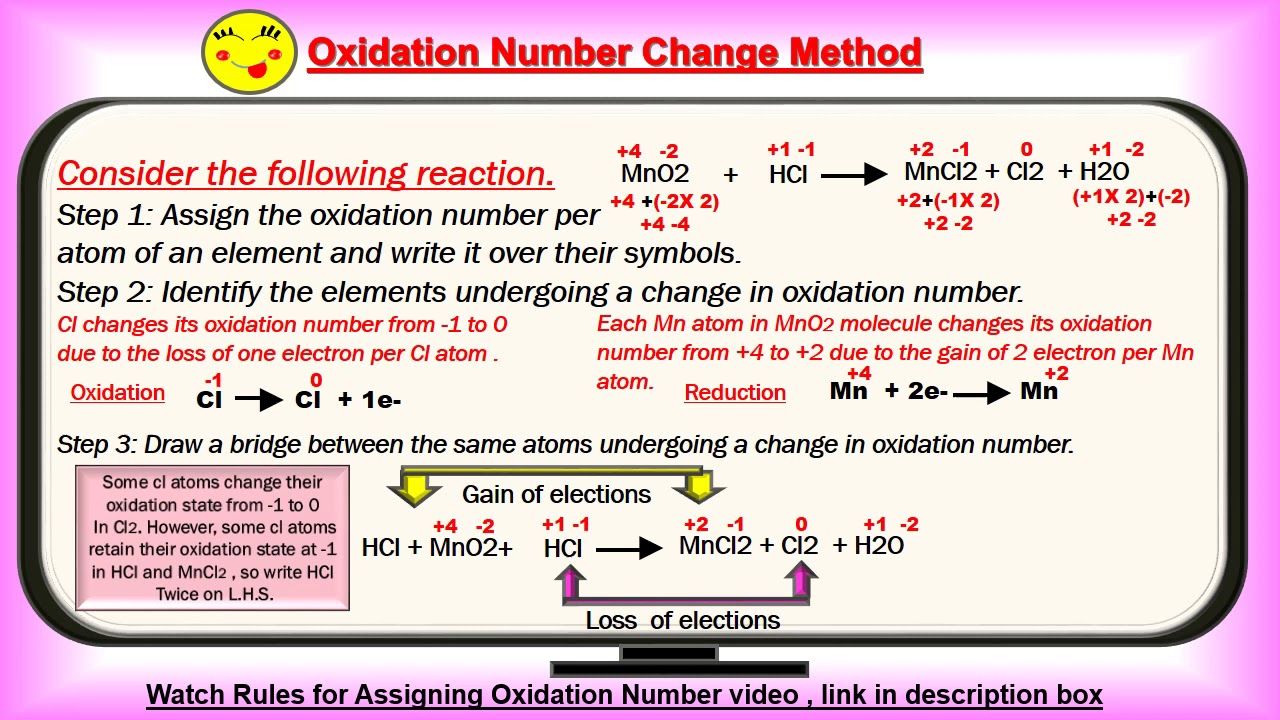
Balancing Redox Reaction By Oxidation Number Change Method Part 2
https://i.ytimg.com/vi/t0arASbWJUU/maxresdefault.jpg
Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation breaking the equation into half reactions adding the electrons balancing the charges with the How to Balance Redox Reactions The redox reaction can be balanced in two ways The change in oxidation numbers of the oxidizing and reducing agents is one technique while the other is
Oct 18 2023 nbsp 0183 32 Steps to Balance the Redox Reaction in Basic Medium by Oxidation Number Method Step 1 Assign the oxidation number to all elements in the reaction to identify atoms Jan 17 2024 nbsp 0183 32 The number of electrons that atoms in a molecule can gain lose or share while establishing chemical interactions with other atoms of a different element is known as the
More picture related to How To Balance Redox Reaction By Oxidation Number Method

Balancing Redox Reactions Half Reaction Method YouTube
https://i.ytimg.com/vi/3AWhDnOPArU/maxresdefault.jpg
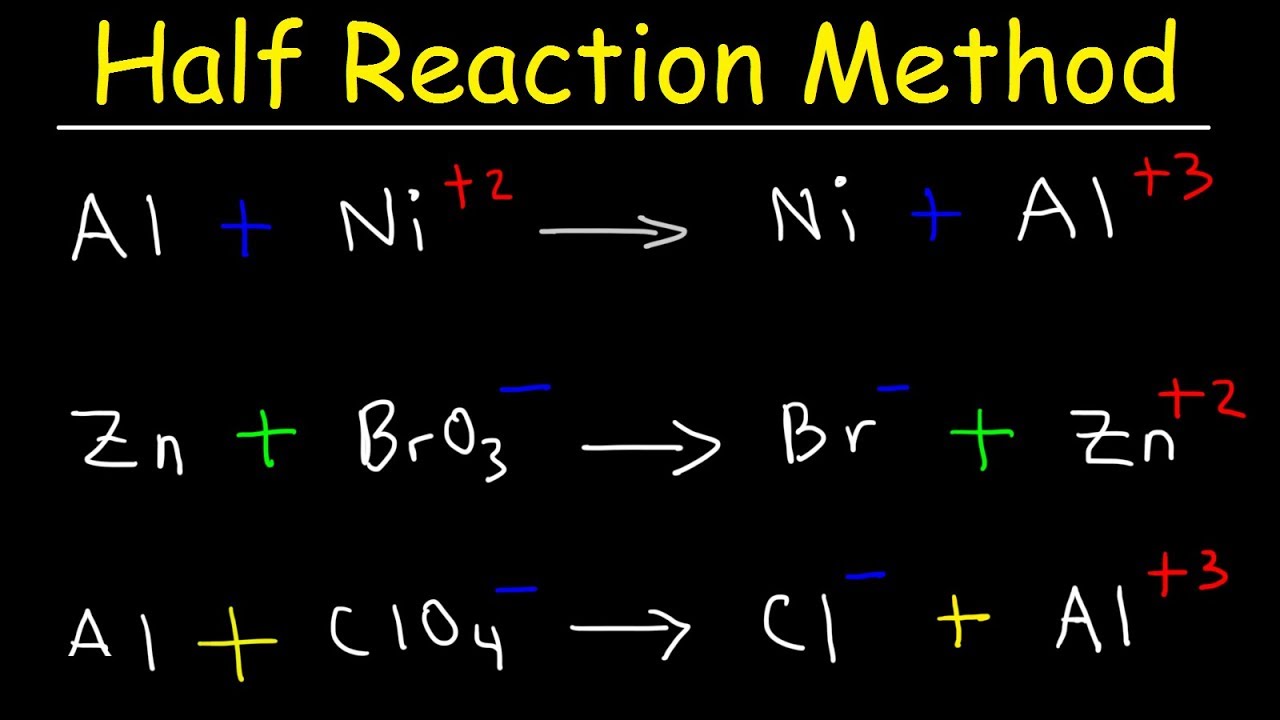
Half Reaction Method Balancing Redox Reactions In Basic Acidic
https://i.ytimg.com/vi/fdbrhQAM9Gw/maxresdefault.jpg

Balancing Redox Reactions Using Oxidation Number Method YouTube
https://i.ytimg.com/vi/Qp1dWUDzzvE/maxresdefault.jpg
Aug 29 2023 nbsp 0183 32 One method used to balance redox reactions is called the Half Equation Method In this method the equation is separated into two half equations one for oxidation and one for There are two common techniques we can use to help us balance redox reactions the oxidation number method and the half reaction method We ll look at the oxidation number method first
First method is oxidation method and second half reaction method or Ion electron method ii The oxidation number for chromium decrease from 6 to 3 So total decrease for two Cr atom is 6 Jan 25 2023 nbsp 0183 32 We learnt how to balance the redox reaction step wise by the oxidation number method and by the ion electron method We also studied that the reaction in the acidic medium

BALANCING OF REDOX REACTIONS By OXIDATION NUMBER METHOD 1 YouTube
https://i.ytimg.com/vi/PlSr0H7J5es/maxresdefault.jpg

Balancing A Redox Reaction Oxidation Number Method YouTube
https://i.ytimg.com/vi/4Rv6kn0qROI/maxresdefault.jpg
How To Balance Redox Reaction By Oxidation Number Method - One way to balance redox reactions is by keeping track of the electron transfer using the oxidation numbers of each of the atoms For the oxidation number change method start with the