How Many Protons Neutrons And Electrons Are In Oxygen Atom Atomic Number Protons Electrons and Neutrons in Oxygen Oxygen is a chemical element with atomic number 8 which means there are 8 protons in its nucleus Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z
An oxygen atom usually has 8 protons 8 neutrons and 8 electrons Looking at the periodic table oxygen has atomic number 8 and atomic weight 15 999 The atomic number tells you how many protons are in the atom s nucleus Oxygen is the 8th element in the periodic table and has a symbol of O and atomic number of 8 It has an atomic weight of 15 999 and a mass number of 16 Oxygen has eight protons and eight neutrons in its nucleus and eight electrons in two shells
How Many Protons Neutrons And Electrons Are In Oxygen Atom
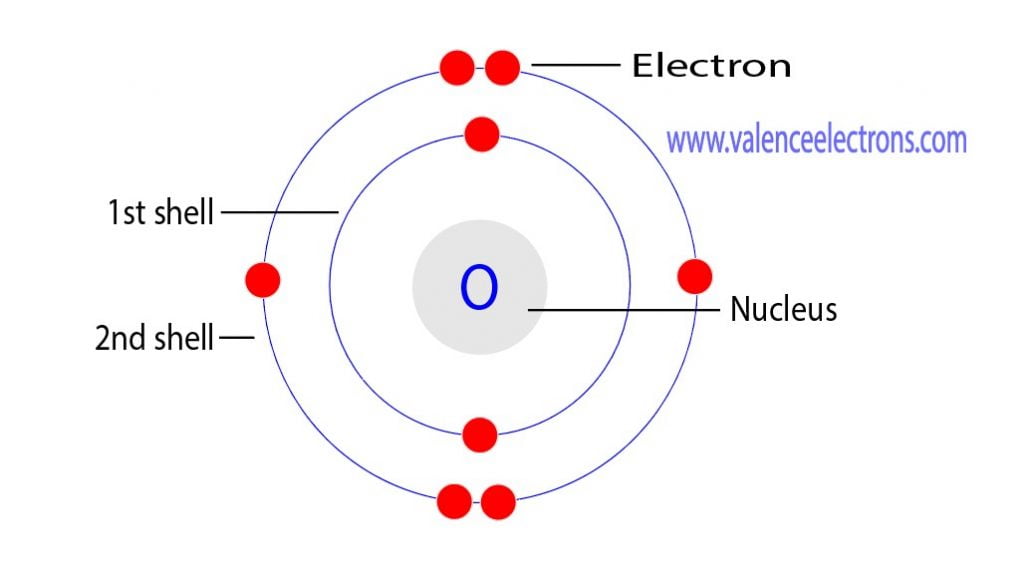
How Many Protons Neutrons And Electrons Are In Oxygen Atom
https://valenceelectrons.com/wp-content/uploads/2021/11/Oxygen-atom-1024x578.jpg

How To Calculate The Number Of Protons Neutrons And Electrons
https://i.ytimg.com/vi/65dDZulPhtg/maxresdefault.jpg
How Many Protons Electrons And Neutrons Are In An Atom Presentation
http://www.sliderbase.com/images/referats/1132b/(1).PNG
Feb 13 2018 nbsp 0183 32 All oxide anions O2 have 8 protons and 10 electrons The number of neutrons depends on the isotope Since you didn t specify which isotope I ll give the answer for all three isotopes The mass number of each isotope is the sum This means that oxygen has 8 protons and 8 electrons In order to get the number of neutrons you take the atomic weight in this case 15 9999 16 and you subtract it by the number of protons 16 8 To get the number of valence electrons just look at the numbers above the oxygen on the periodic table Oxygen is in the 6A group which means that
Nov 21 2020 nbsp 0183 32 Oxygen is a chemical element with atomic number 8 which means there are 8 protons and 8 electrons in the atomic structure The chemical symbol for Oxygen is O The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons The nucleus is composed of protons and neutrons Every oxygen atom has 8 protons and 8 electrons Oxygen has a few isotopes see above the main natural ones on Earth have 8 neutrons about 99 8 percent of oxygen atoms 9 neutrons about 0 04 percent of oxygen atoms and 10 neutrons about 0 2 percent of oxygen atoms
More picture related to How Many Protons Neutrons And Electrons Are In Oxygen Atom

Oxygen Shells Oxygen Atom Chemistry Projects
https://i.pinimg.com/736x/d0/73/cd/d073cd7217be6e43de5b390b8dbd2856--grade--atoms.jpg

Oxygen Atomic Structure Stock Image C018 3689 Science Photo
https://i.pinimg.com/originals/b3/c6/a2/b3c6a2047a526fcbe5d881d66d263de8.jpg

Protons Neutrons Electrons For Oxygen O O2
https://i0.wp.com/valenceelectrons.com/wp-content/uploads/2023/03/Oxygen-protons-neutrons-electrons.jpg
Aug 26 2024 nbsp 0183 32 Some oxygen atoms have 9 neutrons while others have 10 neutrons Oxygen atoms with 9 neutrons would have a mass number of 17 8 mathrm p 9 mathrm n 0 meaning they would have a mass of about 17 amu Apr 28 2014 nbsp 0183 32 The key properties of an atom like oxygen are described by the Atomic Number which is 8 for Oxygen and the total number of nucleons which is 16 for Oxygen The atomic number gives the number of protons in the atom
Aug 22 2024 nbsp 0183 32 Oxygen is the 8th element of the periodic table so its atomic number is 8 Therefore An oxygen atom has eight protons eight neutrons and eight electrons May 21 2024 nbsp 0183 32 An atom of oxygen has 8 protons 8 neutrons and 8 electrons The number of protons determines the element s identity and is equal to the atomic number while the number of neutrons can vary

The Number Of Protons Neutrons And Electrons YouTube
https://i.ytimg.com/vi/CEJ8WoNFFSI/maxresdefault.jpg
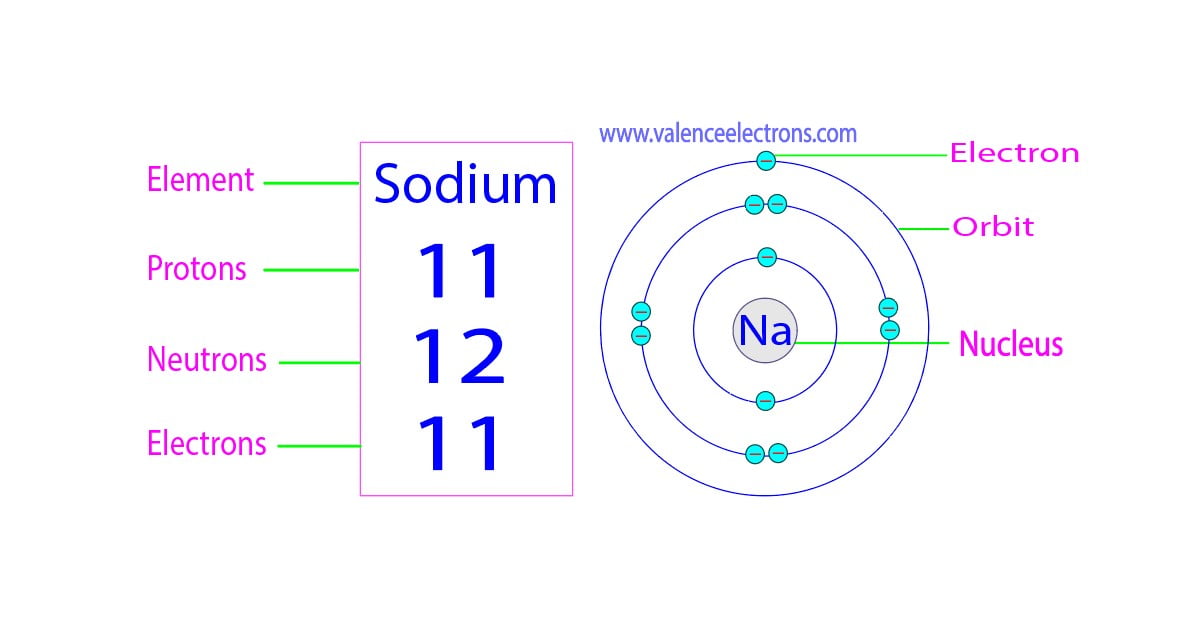
How Many Protons Neutrons And Electrons Does Sodium Have
https://valenceelectrons.com/wp-content/uploads/2022/06/Sodium-protons-neutrons-electrons.jpg
How Many Protons Neutrons And Electrons Are In Oxygen Atom - Every oxygen atom has 8 protons and 8 electrons Oxygen has a few isotopes see above the main natural ones on Earth have 8 neutrons about 99 8 percent of oxygen atoms 9 neutrons about 0 04 percent of oxygen atoms and 10 neutrons about 0 2 percent of oxygen atoms
.PNG)