How Many Protons Neutrons And Electrons Are In Magnesium Protons and Neutrons in Magnesium Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z
May 25 2023 nbsp 0183 32 Magnesium has 12 protons 12 neutrons and 12 electrons But how will you find the number of protons neutrons and electrons in Magnesium Mg Well it is very easy to find the protons neutrons and electrons of magnesium atom Magnesium is the 12th element in the periodic table and has a symbol of Mg and atomic number of 12 It has an atomic weight of 24 305 and a mass number of 24 Magnesium has twelve protons and twelve neutrons in its nucleus and twelve electrons in three shells
How Many Protons Neutrons And Electrons Are In Magnesium
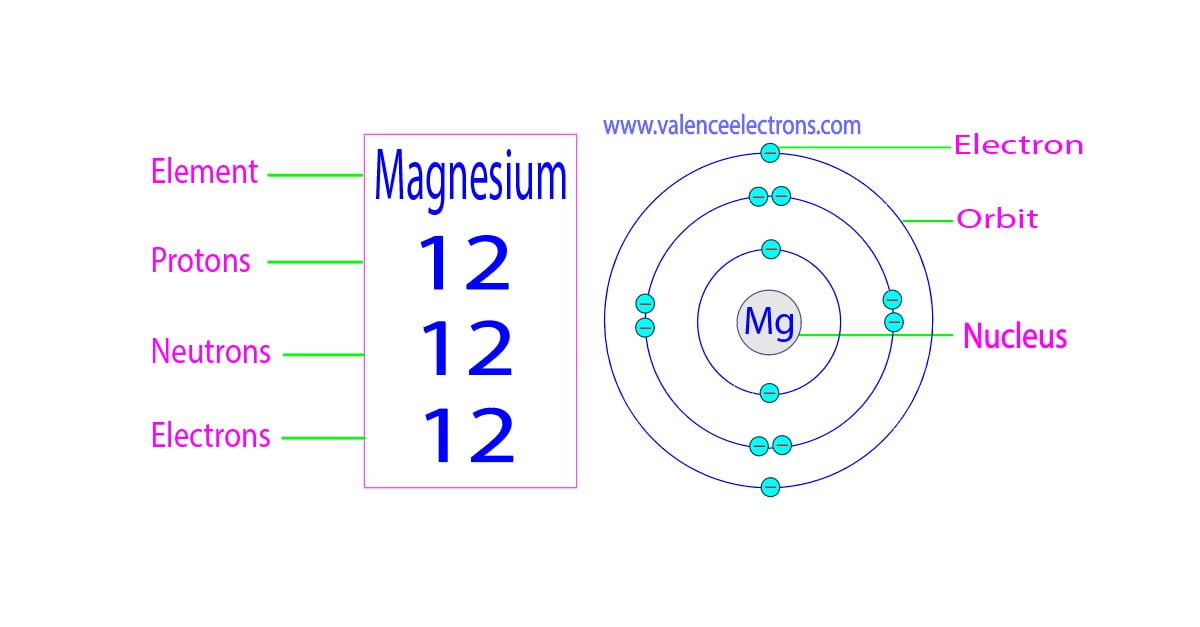
How Many Protons Neutrons And Electrons Are In Magnesium
https://valenceelectrons.com/wp-content/uploads/2022/06/Magnesium-protons-neutrons-electrons.jpg
Solved How Many Protons Neutrons And Electrons Are There Course Hero
https://www.coursehero.com/qa/attachment/25879399/
How Many Protons Electrons And Neutrons Are In An Atom Presentation
http://www.sliderbase.com/images/referats/1132b/(1).PNG
Magnesium has 12 protons and 12 electrons The first electron shell of a Bohr model holds 2 electrons So far 10 of magnesium s 12 electrons have been used so only 2 remain The remaining 2 are placed in the third electron shell which is full when it holds 8 electrons Magnesium is the 12th element of the periodic table so its atomic number is 12 So a magnesium atom has twelve protons twelve neutrons and twelve electrons
Number of protons 12 p Number of neutrons 12 n 0 Number of electrons 12 e Jan 7 2025 nbsp 0183 32 Magnesium a lightweight metal comprises 12 protons 12 neutrons and 12 electrons From the periodic table find the atomic number of magnesium The atomic number of magnesium is 12 Hence magnesium has a total of 12 protons The atomic mass of magnesium is 24 305 so we ll take the roundup value as 24 And the atomic number of magnesium is 12
More picture related to How Many Protons Neutrons And Electrons Are In Magnesium
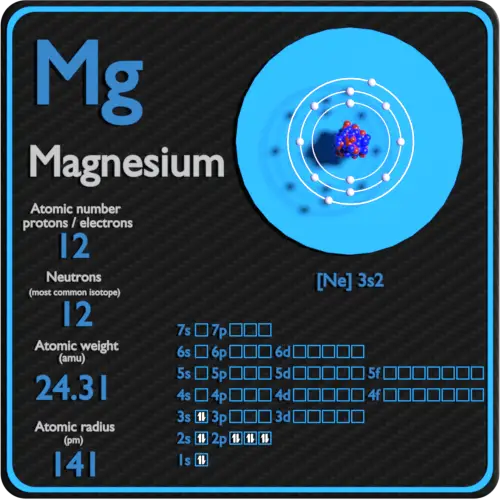
Magnesium Periodic Table And Atomic Properties
https://material-properties.org/wp-content/uploads/2020/09/Magnesium-protons-neutrons-electrons-configuration.png

Periodic Table Element Proton Neutron Electron Periodic Table Printable
https://i0.wp.com/www.periodictableprintable.com/wp-content/uploads/2022/07/periodic-table-of-elements-list-with-protons-neutrons-and-electrons-scaled.jpg?resize=1536%2C1164&ssl=1
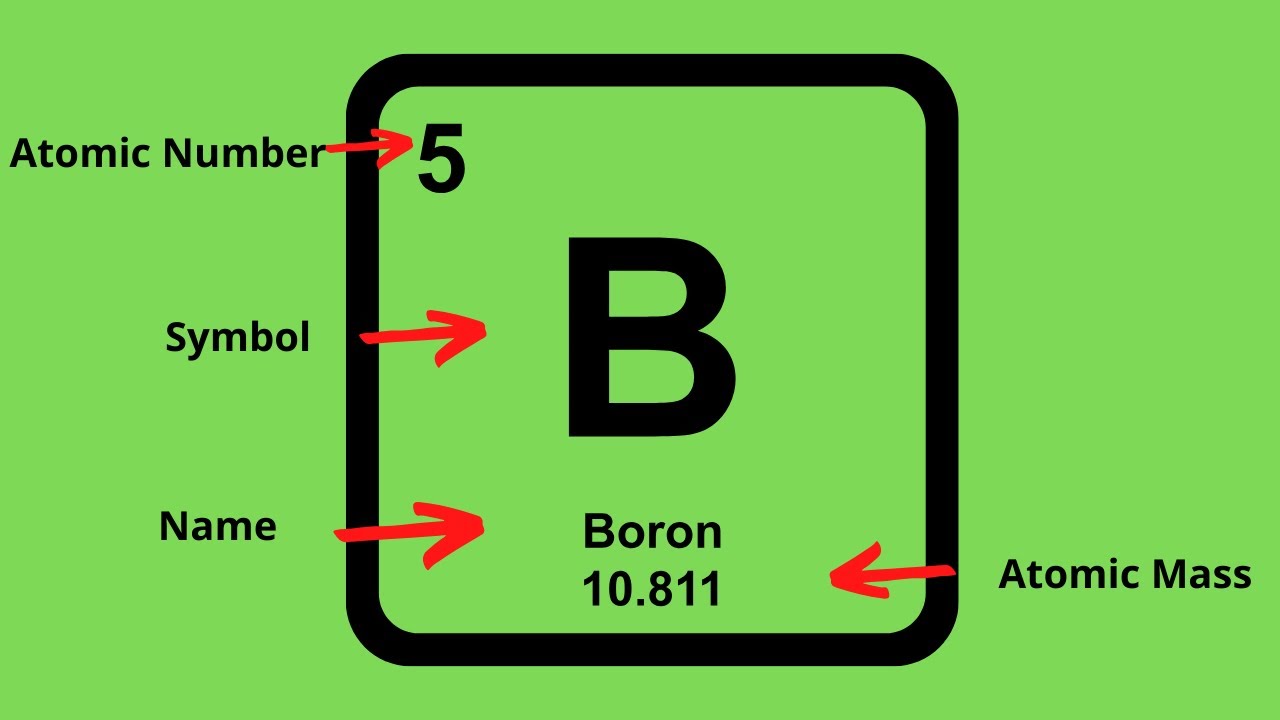
How To Find The Protons Neutrons And Electrons Of An Element On The
https://i.ytimg.com/vi/yooYnW8uhHk/maxresdefault.jpg
Name Magnesium Symbol Mg Atomic Number 12 Atomic Mass 24 305 amu Melting Point 650 0 176 C 923 15 K 1202 0 176 F Boiling Point 1107 0 176 C 1380 15 K 2024 6 176 F Number of Protons Electrons 12 Number of Neutrons 12 Classification Alkaline Earth Crystal Structure Hexagonal Density 293 K 1 738 g cm 3 Color grayish Atomic Structure The most common and stable type of magnesium atom found in nature has 12 protons 12 neutrons and 12 electrons which have a negative charge Atoms of the same element with different neutron counts are known as isotopes
Jun 15 2024 nbsp 0183 32 For instance magnesium has 12 protons making it an element with the atomic number 12 This means that every magnesium atom contains 12 protons 12 electrons and 12 neutrons The number of protons in an atom defines the element while the number of neutrons determines the isotope Sep 28 2020 nbsp 0183 32 In 24 Mg 2 there are 12 protons 12 neutrons and 10 electrons The protons come from the atomic number of magnesium while the neutrons are calculated from the mass number The electrons are determined by the ion s charge which indicates the loss of 2 electrons from the neutral atom

How Many Protons Neutrons And Electrons Does Chlorine Have 2023
https://i0.wp.com/valenceelectrons.com/wp-content/uploads/2022/06/Chlorine-protons-neutrons-electrons.jpg
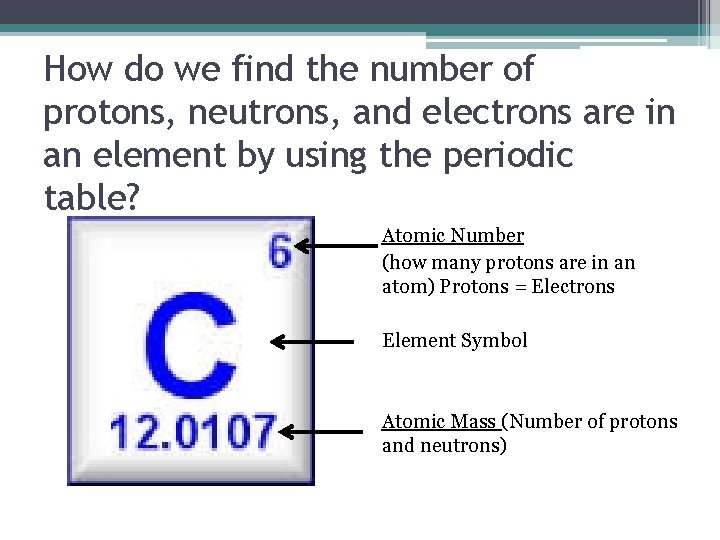
Introduction To Basic Chemistry Protons Neutrons Electrons And
https://slidetodoc.com/presentation_image/54bc97098b887316c5ead05b592cc973/image-14.jpg
How Many Protons Neutrons And Electrons Are In Magnesium - May 22 2024 nbsp 0183 32 Magnesium has 12 protons and 12 electrons in the ground state The number of protons p in the nuclei of an element s atoms is the atomic number of the element In this case the atomic
.PNG)