How Many Protons And Neutrons Does Sodium 23 Have Dec 8 2020 nbsp 0183 32 Sodium 23 is composed of 11 protons 12 neutrons and 11 electrons Acute neutron radiation exposure e g from a nuclear criticality accident converts some of the stable 23Na
Sodium 23 is a natural and stable isotope of the chemical element sodium which in addition to the element specific 11 protons has 12 neutrons in the atomic nucleus resulting in the mass number 23 23 Na is the only stable nuclide of Sodium Na has atomic number of 11 and mass number 23 We know that for a neutral atom Atomic number Z no of proton no of electron No of neutron Mass number Z
How Many Protons And Neutrons Does Sodium 23 Have

How Many Protons And Neutrons Does Sodium 23 Have
https://i.pinimg.com/originals/cf/fc/58/cffc585c3d05bf0729ecb501f068e7c7.png
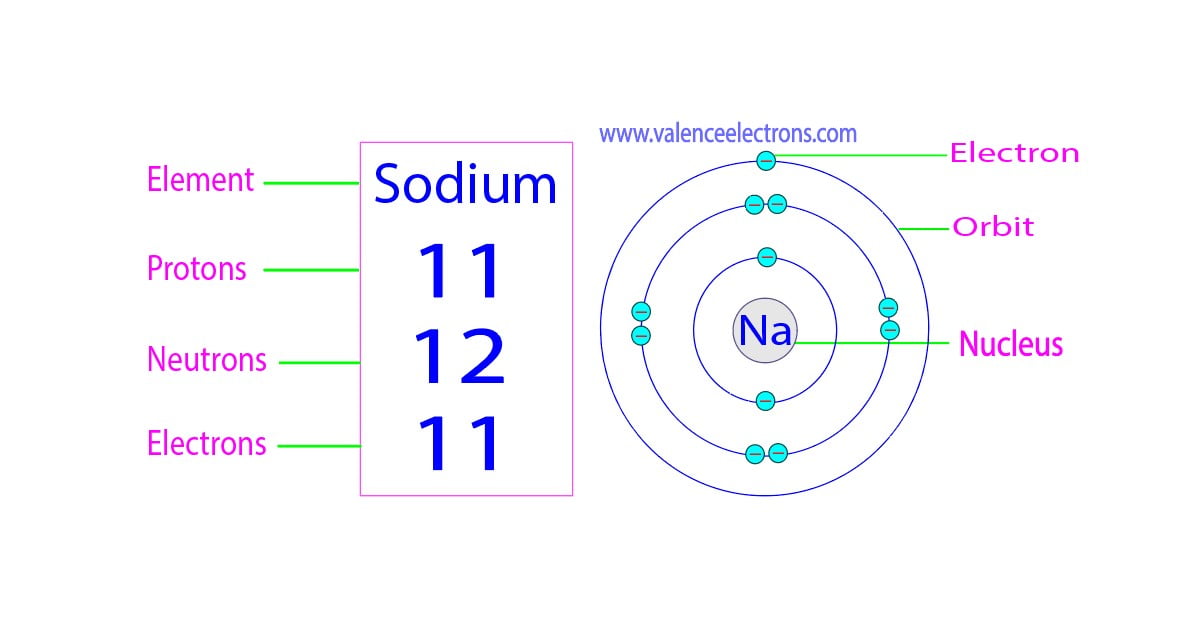
How Many Protons Neutrons And Electrons Does Sodium Have
https://valenceelectrons.com/wp-content/uploads/2022/06/Sodium-protons-neutrons-electrons.jpg

Periodic Table Sodium Protons Neutrons Electrons 2023 Periodic Table
https://i0.wp.com/www.periodictableprintable.com/wp-content/uploads/2022/05/sodium-protons-neutrons-electrons-electron-configuration.png
Sep 7 2023 nbsp 0183 32 The mass number of an atom is the sum of its protons and neutrons In the case of sodium 23 the mass number is 23 which means it consists of 23 nucleons Protons in Sodium Atomic number is equal to the number of protons hence sodium atoms have 11 protons in the nucleus Also the atom has a mass number equal to the number of protons and neutrons
Sodium is the 11th element in the periodic table and has a symbol of Na and atomic number of 11 It has an atomic weight of 22 98977 and a mass number of 23 Sodium has eleven protons and Mar 8 2024 nbsp 0183 32 Hence sodium contains 12 neutrons 11 protons and 11 electrons A neutron is a neutral subatomic particle that in conjunction with protons makes up the nucleus of every atom except ordinary hydrogen whose nucleus has
More picture related to How Many Protons And Neutrons Does Sodium 23 Have
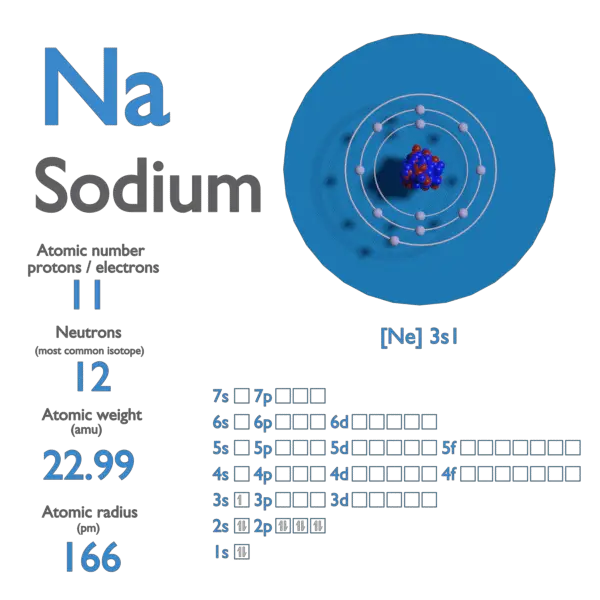
Sodium Atomic Number Atomic Mass Density Of Sodium Nuclear
https://www.nuclear-power.com/wp-content/uploads/2021/11/atomic-number-density-atomic-mass-Sodium.png

Sodium Atom Protons Neutrons Electrons
https://d1avenlh0i1xmr.cloudfront.net/a3108d07-47d4-4404-a645-de46563d93c2/17.-sodium-teachoo-01.png
How Many Protons Neutrons And Electrons Does Gold Have
https://valenceelectrons.com/wp-content/uploads/2022/08/Gold-protons-neutrons-electrons.jpg
Oct 9 2019 nbsp 0183 32 How many protons neutrons and electrons does an atom of sodium 23 have An atomic number of 11 means this atom will have 11 protons A mass number of 23 means 23 The difference between the mass number of the sodium atom and the number of protons is twelve Therefore a sodium atom has twelve neutrons The number of neutrons depends on
May 18 2021 nbsp 0183 32 How many protons neutrons and electrons does an atom of sodium 23 have An atomic number of 11 means this atom will have 11 protons A mass number of 23 means 23 For sodium that means there are 11 protons and 11 electrons Since we know that there are 11 protons there must be 12 neutrons for the mass number to equal 23 23 11 12 Answered by
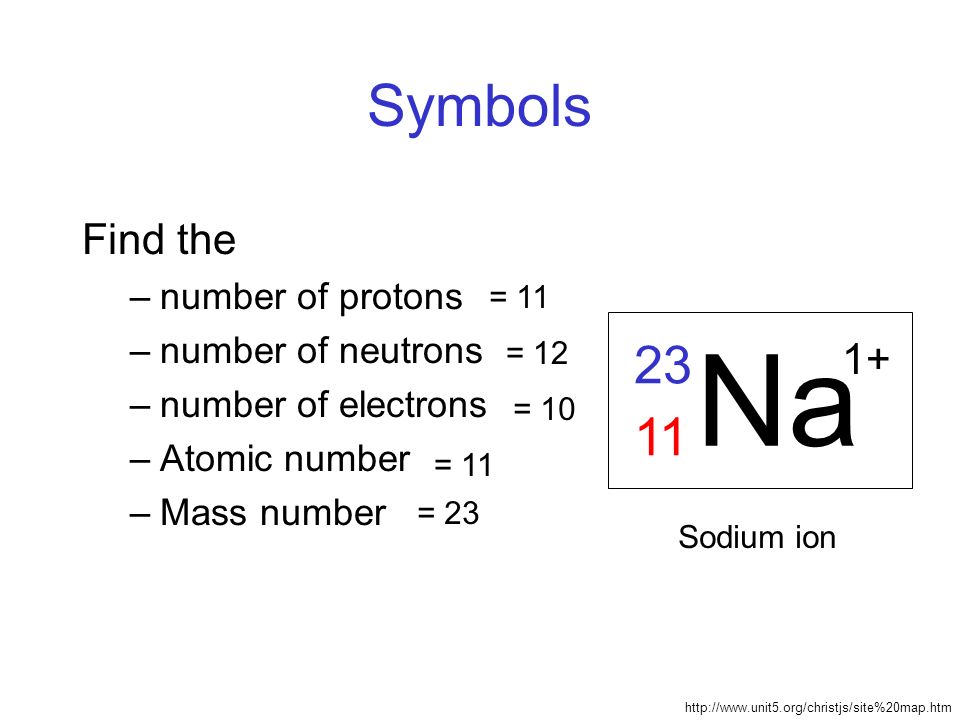
Sodium Mass Number And Atomic Number
https://periodictable.me/wp-content/uploads/2018/08/NaSymbolsFindthe1numberofprotonsnumberofneutrons.jpg?8a3680&8a3680
Understanding Protons Electrons And Neutrons
https://lh6.googleusercontent.com/proxy/cHmJ4HVruNArIS-g6hNX89WoA7T5gTJJW20eAPyow--172JAhLyOQCyxKdJr3UfUzF_joaggZotDXoiZhW_KT36u-nz_BwYXA2Ja7QWs2wxcbBSZT9x-3qxMsVQkRRJbVs5MypfI8nI84w=s0-d
How Many Protons And Neutrons Does Sodium 23 Have - Sodium is the 11th element in the periodic table and has a symbol of Na and atomic number of 11 It has an atomic weight of 22 98977 and a mass number of 23 Sodium has eleven protons and