How Do You Calculate Average Atomic Mass Of Isotopes Sep 20 2022 nbsp 0183 32 The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element The sample problem below demonstrates how
6 days ago nbsp 0183 32 To calculate the average atomic mass you may use the simple formula AM f 215 m f 215 m f n 215 m n where AM Average atomic mass f n Natural abundance of nth The average atomic mass is the average mass of all the isotopes that compose that element weighted based on the natural abundance of each isotope So how do we calculate the
How Do You Calculate Average Atomic Mass Of Isotopes

How Do You Calculate Average Atomic Mass Of Isotopes
http://www.wikihow.com/images/f/f0/Calculate-Atomic-Mass-Step-7-Version-3.jpg
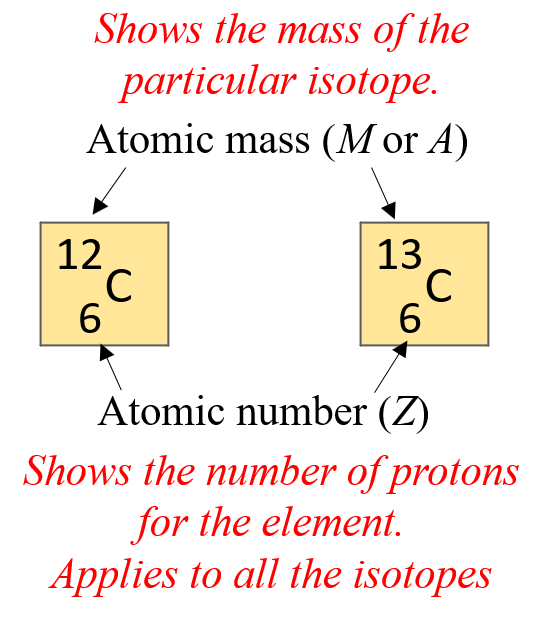
What Are Isotopes Chemistry Steps
https://general.chemistrysteps.com/wp-content/uploads/2022/11/atomic-number-and-mass-number.png
Isotopes
http://www.sliderbase.com/images/referats/1140b/(23).PNG
Average Atomic Mass Mass of Isotope 1 x Fractional Abundance of Isotope 1 Mass of Isotope 2 x Fractional Abundance of Isotope 2 The average atomic mass has been How do you calculate the average atomic mass To calculate the average atomic mass follow these steps Identify isotopes List all naturally occurring isotopes of the element Gather data
Dec 12 2024 nbsp 0183 32 Enter the percentage abundance and mass of up to 5 different isotopes into the average atom mass calculator The calculator will display the average atomic mass of the The average atomic mass is calculated as Average Atomic Mass 34 96885 215 0 7578 36 96590 215 0 2422 Average Atomic Mass 26 50 8 95 35 45 amu
More picture related to How Do You Calculate Average Atomic Mass Of Isotopes
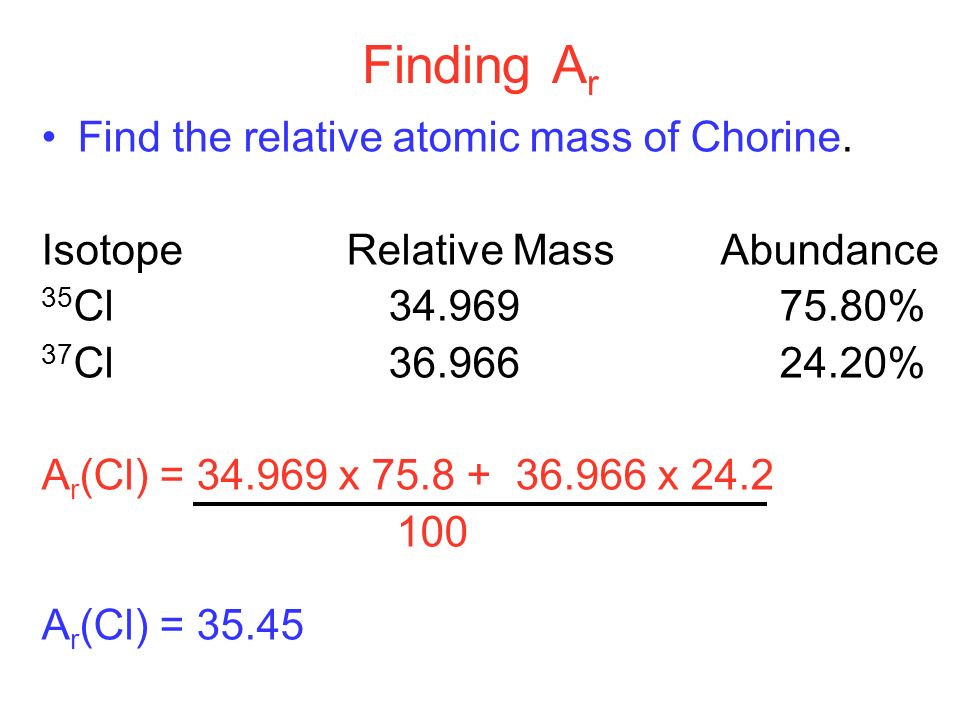
Way To Find Atomic Mass Of Elements Dynamic Periodic Table Of
http://periodictable.me/wp-content/uploads/2018/08/slide_33.jpg
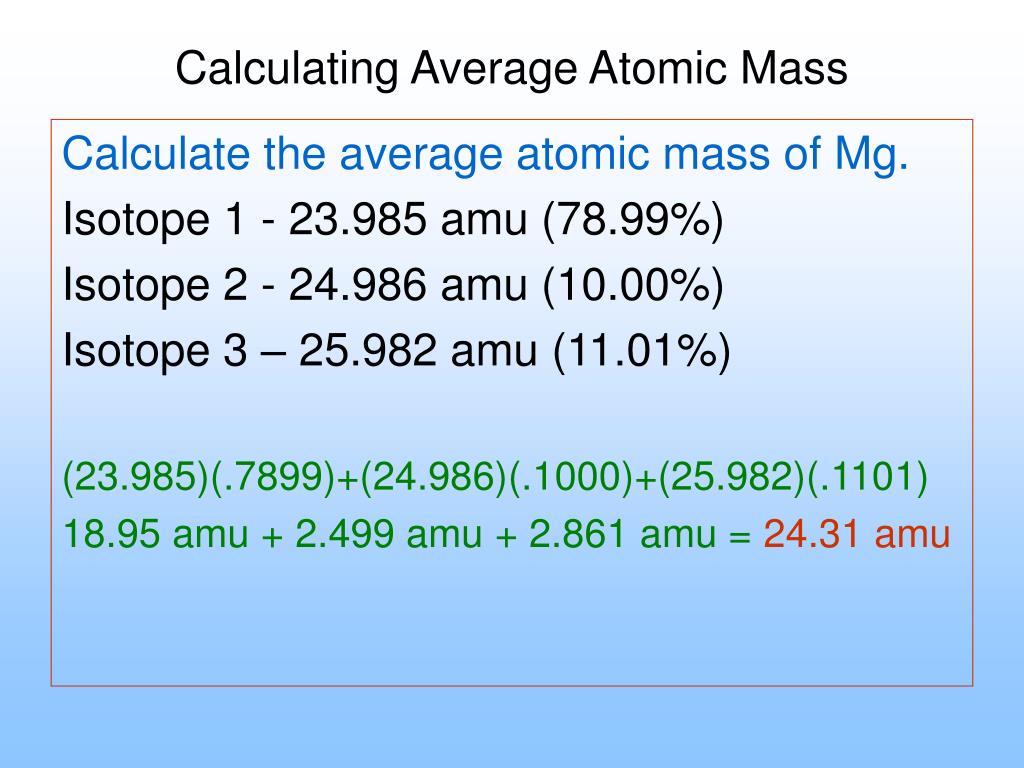
Isotopes And Average Atomic Masses Worksheet
https://image1.slideserve.com/3225780/calculating-average-atomic-mass1-l.jpg
Solved Gallium Has Two Naturally Occurring Isotopes Ga 69Ga 69 With
https://www.coursehero.com/qa/attachment/34235414/
If given the atomic mass of the isotopes of an element as well as their relative abundances we can follow simple steps to calculate the atomic weight Using isotope abundance to calculate Dec 29 2017 nbsp 0183 32 How do you calculate atomic mass To calculate the atomic mass of an element we have to calculate how much each isotope contributes to the mass of the atom To
To calculate atomic mass you ll need two pieces of information the masses of each isotope and their isotopic abundances You can find these values on a periodic table or in reference books The formula for relative atomic mass is A r average mass of isotopes of the element Example Given that the percentage abundance of is 75 and that of is 25 calculate the A r of
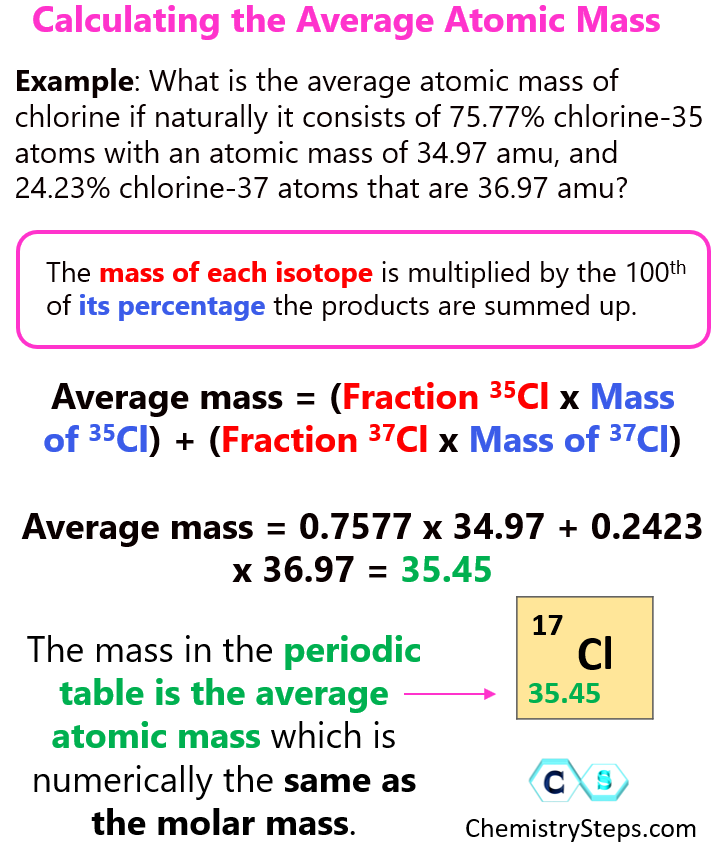
How To Calculate The Average Atomic Mass Chemistry Steps
https://general.chemistrysteps.com/wp-content/uploads/2023/01/How-To-Calculate-The-Average-Atomic-Mass.png

Calculating Percent Abundance And Average Atomic Mass YouTube
https://i.ytimg.com/vi/WkQWVyohYxA/maxresdefault.jpg
How Do You Calculate Average Atomic Mass Of Isotopes - Dec 12 2024 nbsp 0183 32 Enter the percentage abundance and mass of up to 5 different isotopes into the average atom mass calculator The calculator will display the average atomic mass of the
.PNG)