How Are Protons And Neutrons Similar And How Are They Different Dec 26 2024 nbsp 0183 32 Protons and neutrons are similar as they are both nucleons located within the nucleus and have comparable masses They differ in charge with protons being positively charged and neutrons being neutral and they play different roles in maintaining nuclear stability
Like protons neutrons are bound into the atom s nucleus as a result of the strong nuclear force Protons and neutrons have approximately the same mass but they are both much more massive than electrons approximately 2 000 times as massive as an electron Neutrons are in every atom with one exception and they are bound together with other neutrons and protons in the atomic nucleus Before we move on we must discuss how the different types of subatomic particles interact with each other
How Are Protons And Neutrons Similar And How Are They Different
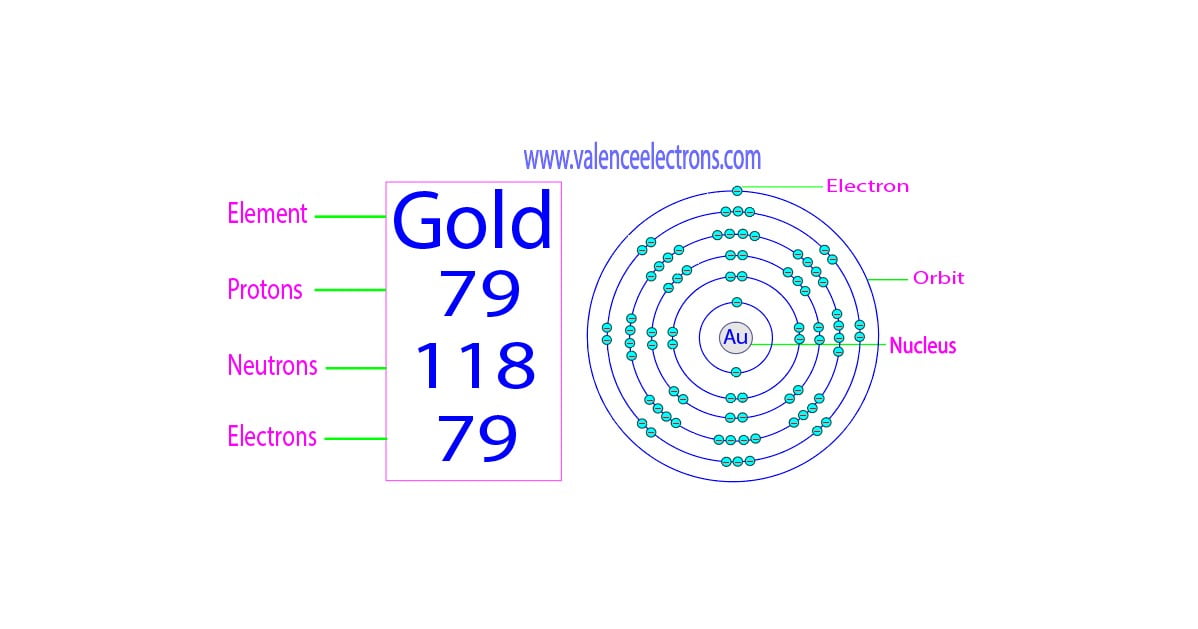
How Are Protons And Neutrons Similar And How Are They Different
https://valenceelectrons.com/wp-content/uploads/2022/08/Gold-protons-neutrons-electrons.jpg
How Are Protons And Neutrons Similar How Are They Di SolvedLib
https://cdn.numerade.com/previews/da99e5f3-6e50-4451-a78a-a30cef1c90ec_large.jpg
Solved How Many Protons Neutrons And Electrons Are There Course Hero
https://www.coursehero.com/qa/attachment/25879399/
Nov 2 2023 nbsp 0183 32 The electromagnetic force pushes the protons apart but the strong force which is greater across short distances pulls them closer In summary protons and neutrons have roughly the same mass and are found in an atom s nucleus Protons have a positive charge while neutrons have no charge Feb 2 2025 nbsp 0183 32 Protons and neutrons are both found in the nucleus of an atom and are similar in mass contributing to atomic mass and identified as nucleons The key differences include protons having a positive charge and determining atomic identity
How are protons and neutrons similar How are they different Both are subatomic particles that reside in an atom s nucleus Both have approximately the same mass Protons are positively charged whereas neutrons are uncharged We have an expert written solution to this problem May 28 2024 nbsp 0183 32 Protons are positively charges and neutrons carry no charge Protons are positively charged particles found in the nucleus of an atom while neutrons are neutral particles also located in the
More picture related to How Are Protons And Neutrons Similar And How Are They Different
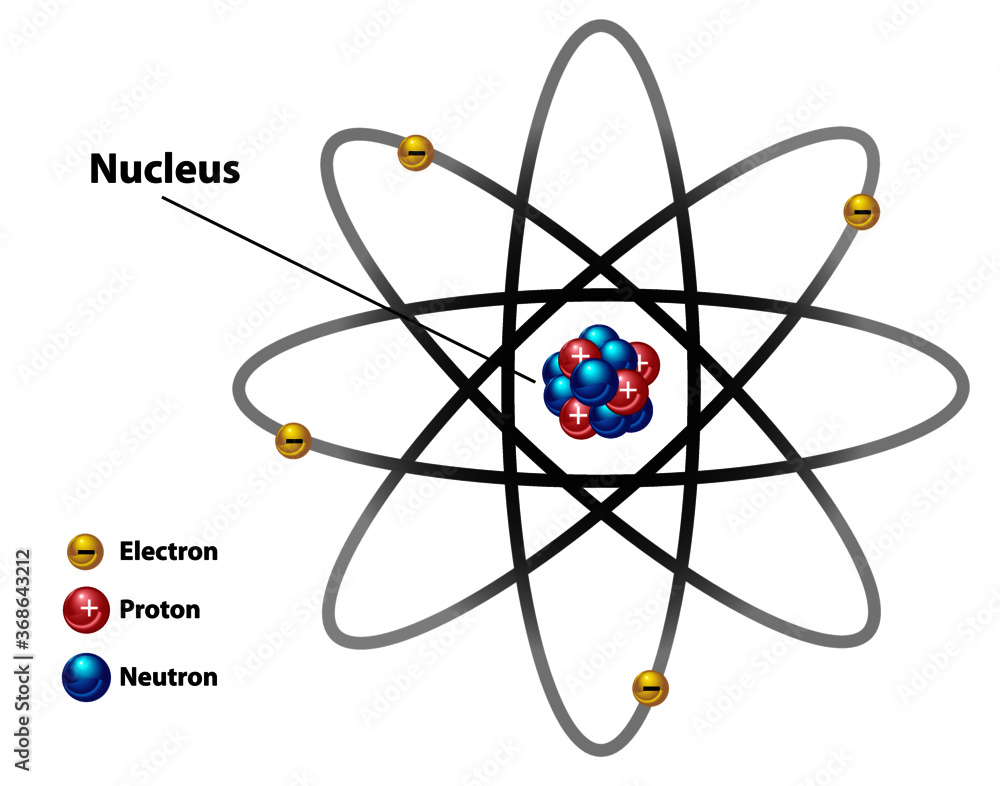
Atomic Nucleus Diagram Labeled With Electron Proton And Neutron
https://as1.ftcdn.net/v2/jpg/03/68/64/32/1000_F_368643212_ovdSJUrD6hM0crl4B03QzcQ55XlyowRS.jpg

Periodic Table Element Proton Neutron Electron Periodic Table Printable
https://i0.wp.com/www.periodictableprintable.com/wp-content/uploads/2022/07/periodic-table-of-elements-list-with-protons-neutrons-and-electrons-scaled.jpg?resize=1536%2C1164&ssl=1
Understanding Protons Electrons And Neutrons
https://lh6.googleusercontent.com/proxy/cHmJ4HVruNArIS-g6hNX89WoA7T5gTJJW20eAPyow--172JAhLyOQCyxKdJr3UfUzF_joaggZotDXoiZhW_KT36u-nz_BwYXA2Ja7QWs2wxcbBSZT9x-3qxMsVQkRRJbVs5MypfI8nI84w=s0-d
Protons and neutrons have roughly the same mass 1 67 x grams This amount of mass is defined by scientists as one atomic mass unit amu or one Dalton Both reside in an atom s nucleus Despite their comparable mass protons are positively charged whereas neutrons are neutral Mar 15 2024 nbsp 0183 32 Protons positively charged particles in an atom s nucleus define an element s identity while neutrons neutral particles contribute to atomic mass but not charge
Differences Protons have a positive charge while neutrons have no charge The number of protons determines the atomic number element identity while the number of neutrons determines the isotope of an element Step 1 2Protons and neutrons are both subatomic particles found in the nucleus of an atom They are similar in that they both have a mass of approximately one atomic mass unit amu and contribute to the overall mass of the atom However they differ in their charge Protons have a positive charge while neutrons have no charge they are neutral

How To Teach Finding Protons Neutrons And Electrons In An Element
https://images.squarespace-cdn.com/content/v1/61a7976ff13b3127ab967596/c3da6672-95e4-4257-b29c-cdd6cc8a8634/1.png

Proton Neutron Electron Chart
https://physfox.s3.eu-west-2.amazonaws.com/!electricity/pne/table-pne.png
How Are Protons And Neutrons Similar And How Are They Different - Feb 2 2025 nbsp 0183 32 Protons and neutrons are both found in the nucleus of an atom and are similar in mass contributing to atomic mass and identified as nucleons The key differences include protons having a positive charge and determining atomic identity