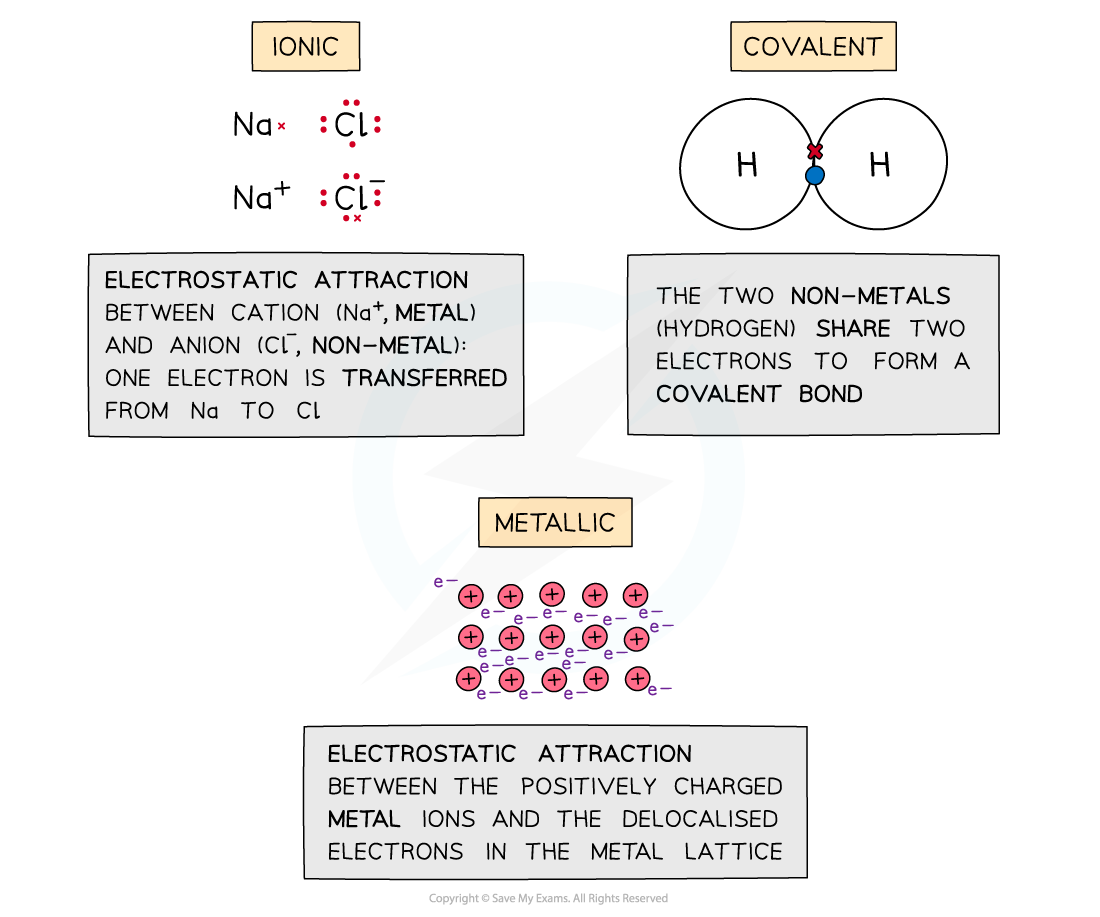
Four Types Of Intramolecular Forces Types of Intramolecular Forces 1 Ionic Bond An ionic bond is formed by the complete transfer of valence electrons between the two atoms This transfer of electrons leads to the formation of two oppositely charged ions The force of attraction between these differently charged ions is responsible to hold the atoms in position
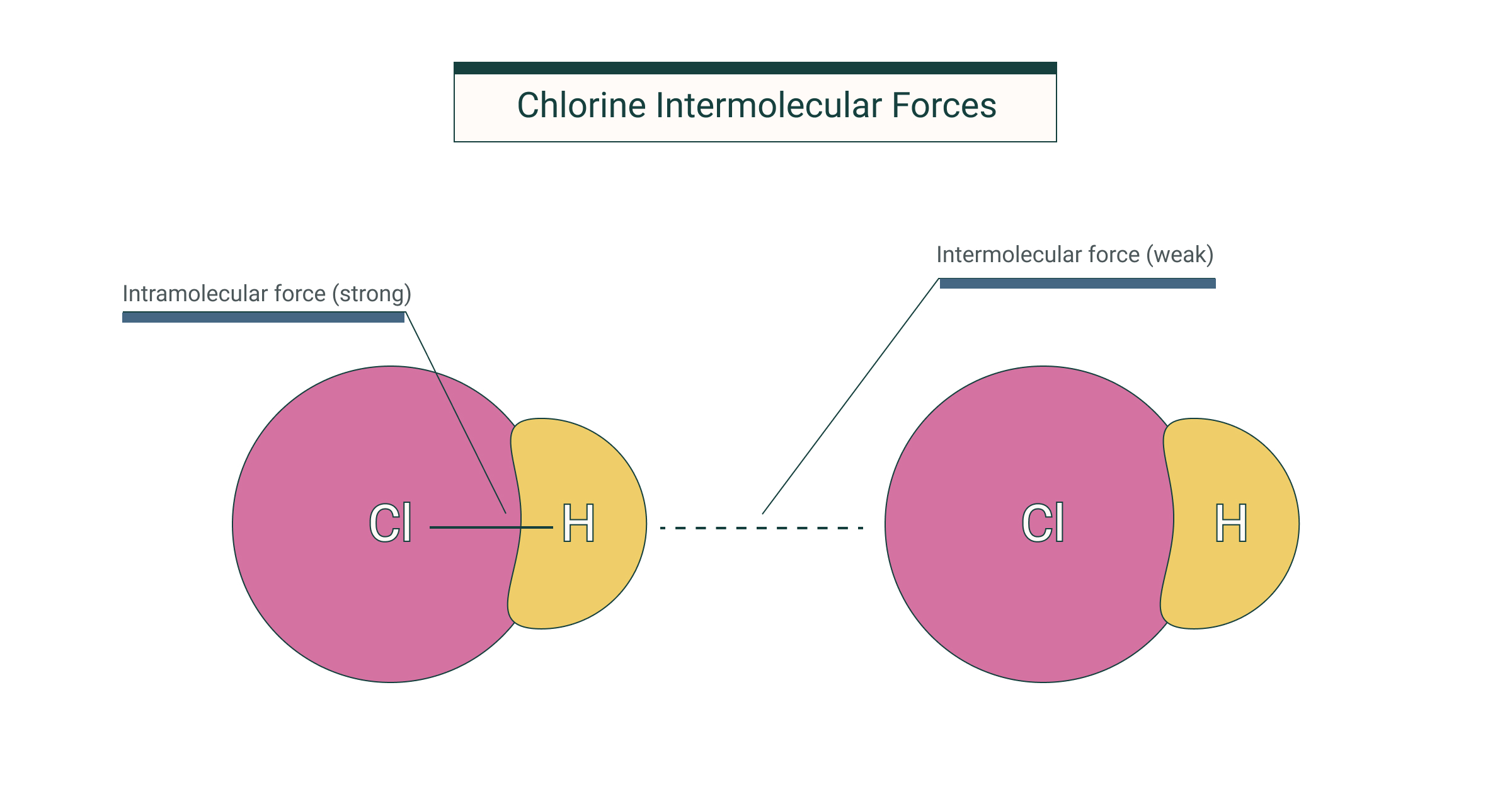
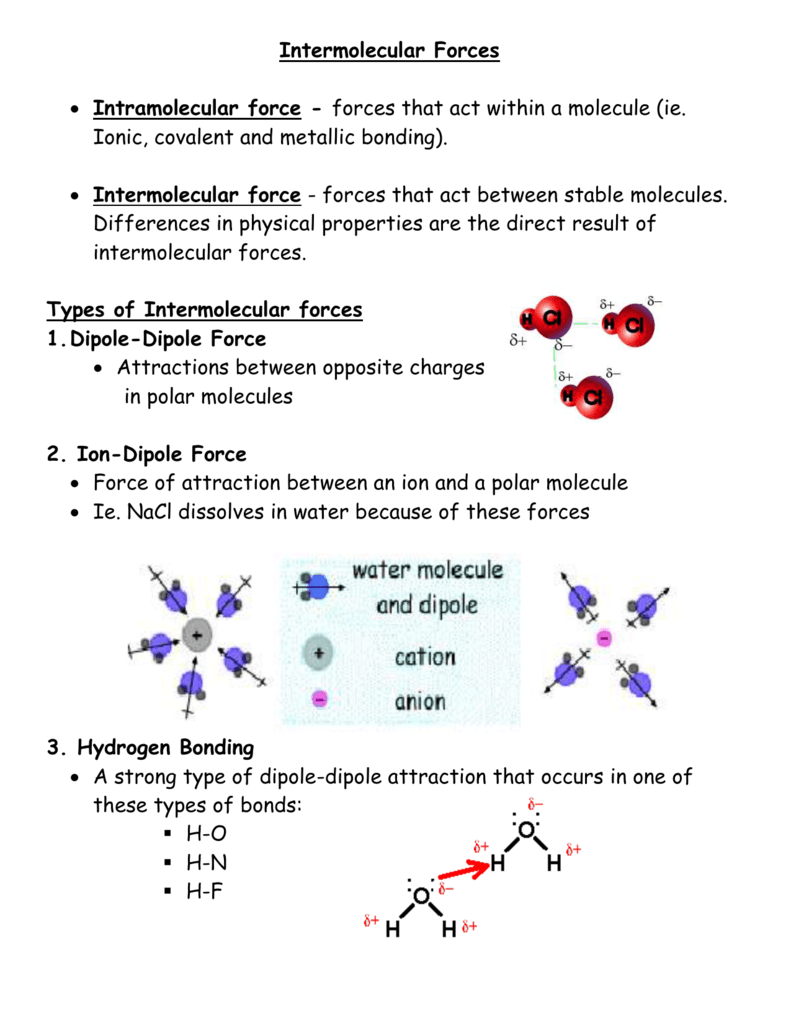
Intramolecular forces are the chemical bonds holding the atoms together in the molecules The three major types of chemical bonds are the metallic bond the ionic bond and the covalent bond Oct 12 2022 nbsp 0183 32 Intramolecular forces act between atoms within a molecule The types of intramolecular forces are ionic bonds covalent bonds and metallic bonds In chemistry intramolecular forces are that hold atoms together in a molecule
Four Types Of Intramolecular Forces
Four Types Of Intramolecular Forces
https://pediaa.com/wp-content/uploads/2017/01/Difference-Between-Intermolecular-and-Intramolecular-Forces-Comparison-Summary-609x1024.png
Intermolecular Forces In Covalent Molecules
https://uploads-ssl.webflow.com/5d2a13e2187e933942be8fc3/5e31031183dd661179515442_27 - Intermolecular forces.jpg
CIE A Level Chemistry 1 3 15 Inter Intramolecular Forces
https://oss.linstitute.net/wechatimg/2022/08/1.3-Chemical-Bonding-Intramolecular-Forces-1.png
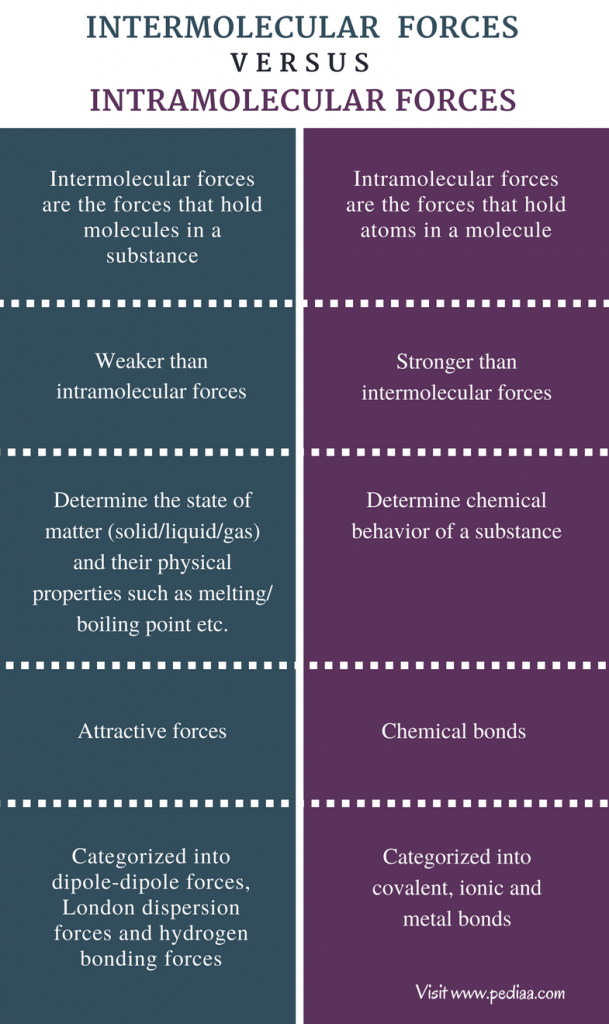
Oct 27 2024 nbsp 0183 32 There are three types of intermolecular forces Induced dipole dipole forces also called van der Waals or London dispersion forces Permanent dipole dipole forces are the attractive forces between two neighbouring molecules with a permanent dipole Intramolecular forces are stronger than the intermolecular forces that govern the interactions between molecules 2 The classical model identifies three main types of chemical bonds ionic covalent and metallic distinguished by the degree of
Dipole Dipole Interactions or Dispersion Forces If two molecules are of comparable size and shape dipole dipole interactions will likely be the dominating force If one molecule is much larger than another dispersion forces will likely determine its physical properties Feb 9 2023 nbsp 0183 32 These forces are known as intermolecular forces There are three main types of intermolecular forces London dispersion forces Dipole dipole attraction Hydrogen bonding
More picture related to Four Types Of Intramolecular Forces

Intramolecular Forces
https://sciencenotes.org/wp-content/uploads/2022/10/Types-of-Intramolecular-Forces.png

Intermolecular Forces Chemistry Video Clutch Prep
https://cdn.clutchprep.com/guide_visuals/inline_images/Sb9OoXBMiTgVoII8mgpNfw.png
Intermolecular Forces And Solubility
https://s3.studylib.net/store/data/007288258_1-b1ddcbba901306f7e889aed102d8a0fe.png
1 day ago nbsp 0183 32 Core Answer Intramolecular forces are the forces within a molecule holding atoms together These include covalent bonds single double and triple ionic bonds and metallic bonds Intermolecular forces are the forces between molecules These include London Dispersion Forces LDFs dipole dipole forces and hydrogen bonds Identify the types of intermolecular forces experienced by specific molecules based on their structures Explain the relation between the intermolecular forces present within a substance and the temperatures associated with changes in its physical state
Jan 15 2017 nbsp 0183 32 The main difference between intermolecular and intramolecular forces is that intermolecular forces exist between the molecules themselves whereas intramolecular forces exist between atoms within a molecule Intermolecular forces also known as intermolecular interactions are the electrostatic forces of attraction between molecules in a compound The intermolecular forces tend to attract the molecules together bring them closer and make the compound stable

What Are The Intermolecular Forces Example
http://slideplayer.com/6977076/24/images/14/Summary+of+Intermolecular+Forces.jpg
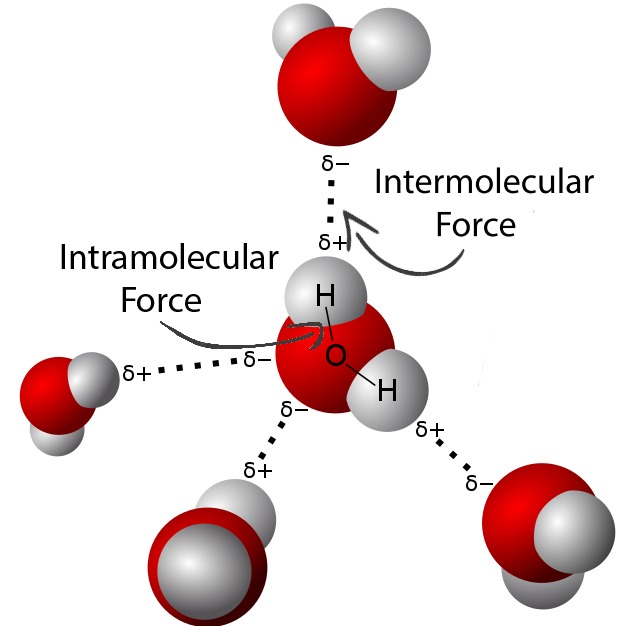
Influence Of Intermolecular Forces ChemTalk
https://chemistrytalk.org/wp-content/uploads/2023/08/Web-capture_7-8-2023_12422_.jpeg
Four Types Of Intramolecular Forces - Jan 15 2024 nbsp 0183 32 Figure PageIndex 4 Intramolecular forces keep a molecule intact Intermolecular forces hold multiple molecules together and determine many of a substance s properties In this section we will discuss the three types of IMF in molecular compounds dipole dipole hydrogen bonding and London dispersion forces