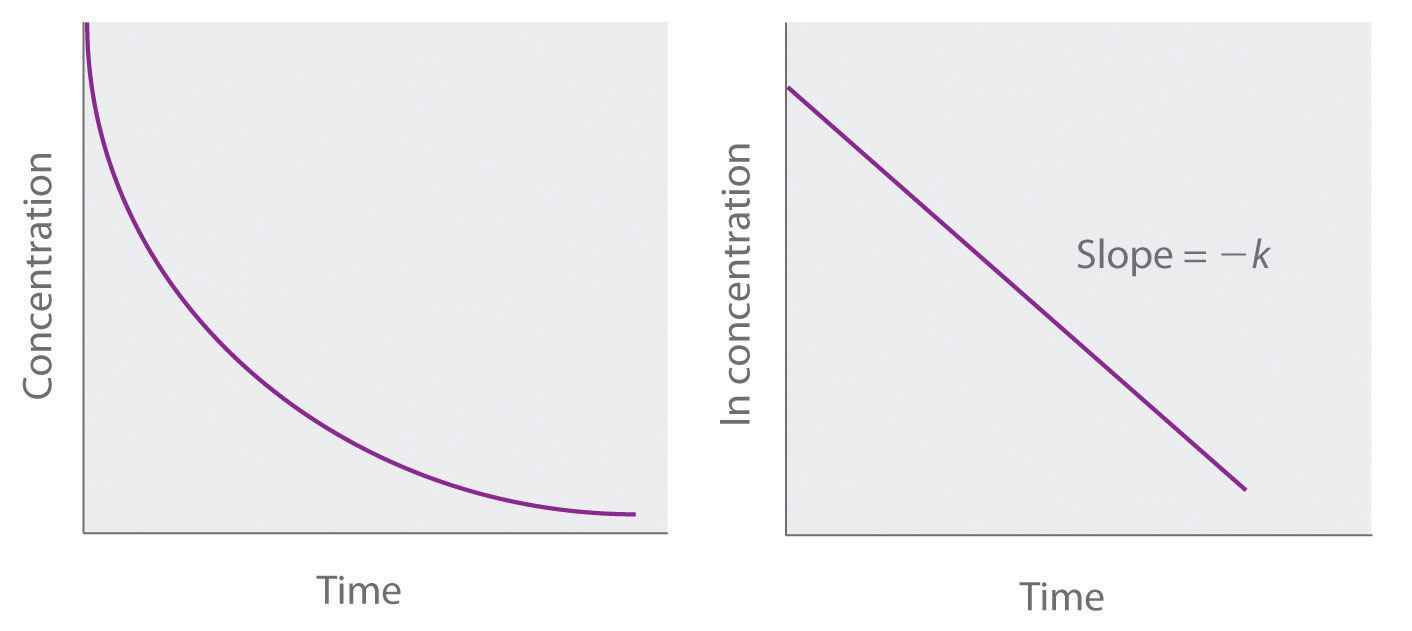
First Order Reaction Graph Between Concentration And Time The concentration v s time graph for a first order reaction is provided below For first order reactions the equation ln A kt ln A 0 is similar to that of a straight line y mx c with slope k
Jul 12 2023 nbsp 0183 32 Figure PageIndex 4 Graphs of a first order reaction The expected shapes of the curves for plots of reactant concentration versus time top and the natural logarithm of With the help of the graph one can determine that the concentration of the reactant decreases exponentially with time which is a characteristic feature of the first order reaction The rate of
First Order Reaction Graph Between Concentration And Time

First Order Reaction Graph Between Concentration And Time
https://i.ytimg.com/vi/toNzhxKKku4/maxresdefault.jpg
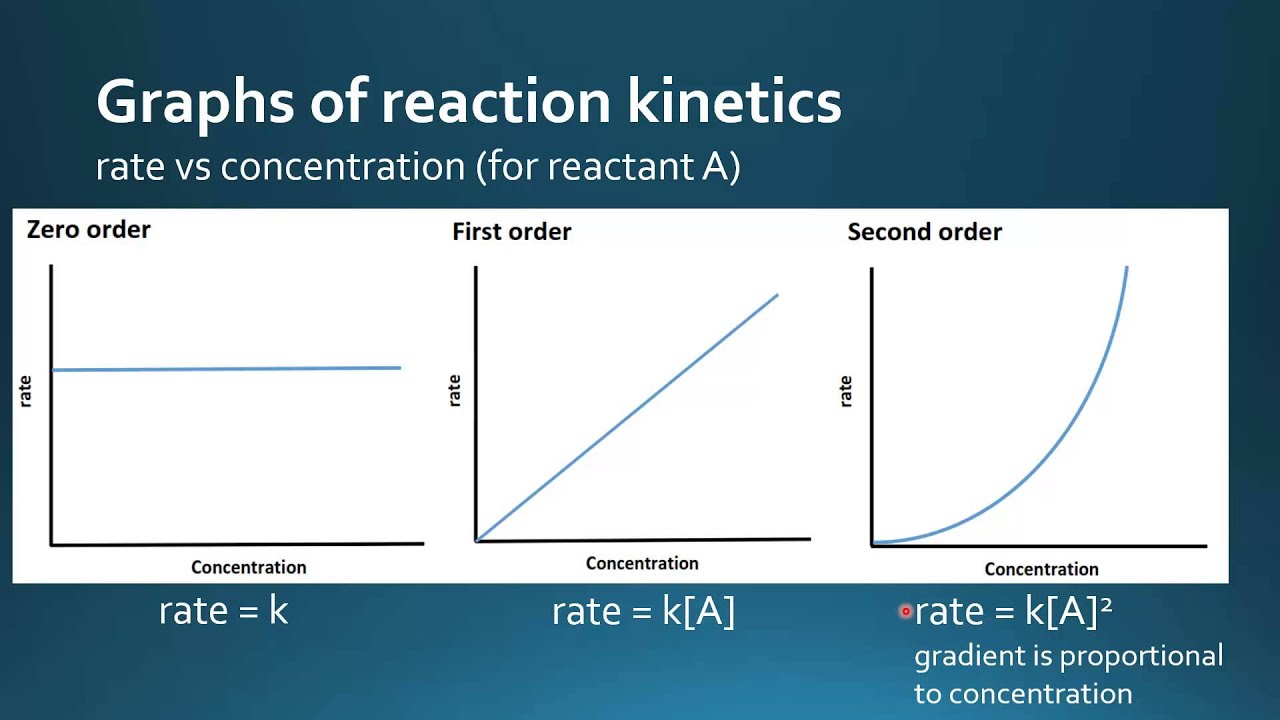
Reactivity 2 2 10 Graphical Representations For Zero First And
https://i.ytimg.com/vi/SuZkqJ79BKk/maxresdefault.jpg

Graph Of Integrated Rate Equation Of Zero Order Reaction chemical
https://i.ytimg.com/vi/BZ8Qwg25srA/maxresdefault.jpg
Feb 13 2023 nbsp 0183 32 For a first order reaction a plot of the natural logarithm of the concentration of a reactant versus time is a straight line with a slope of k For a second order reaction a plot of the inverse of the concentration of a reactant Aug 23 2024 nbsp 0183 32 Concentration Time Graph For a First Order Reaction A graph showing the constant half life of a first order reaction Radioactive decays are good examples of reactions
Concentration vs Time First Order Reaction This graph shows the plot of the natural logarithm of concentration versus time for a first order reaction The decomposition of a pollutant in water Concentration time graph for the reaction 2NH3 N2 3H2 2 NH 3 N 2 3 H 2 Source OpenStax Chemistry Atoms First 2e In general the rate of reaction will vary as a function of the concentration of reactants and hence the rate of
More picture related to First Order Reaction Graph Between Concentration And Time

Zeroth Order
https://i.ytimg.com/vi/7I0Xg92_eA4/maxresdefault.jpg
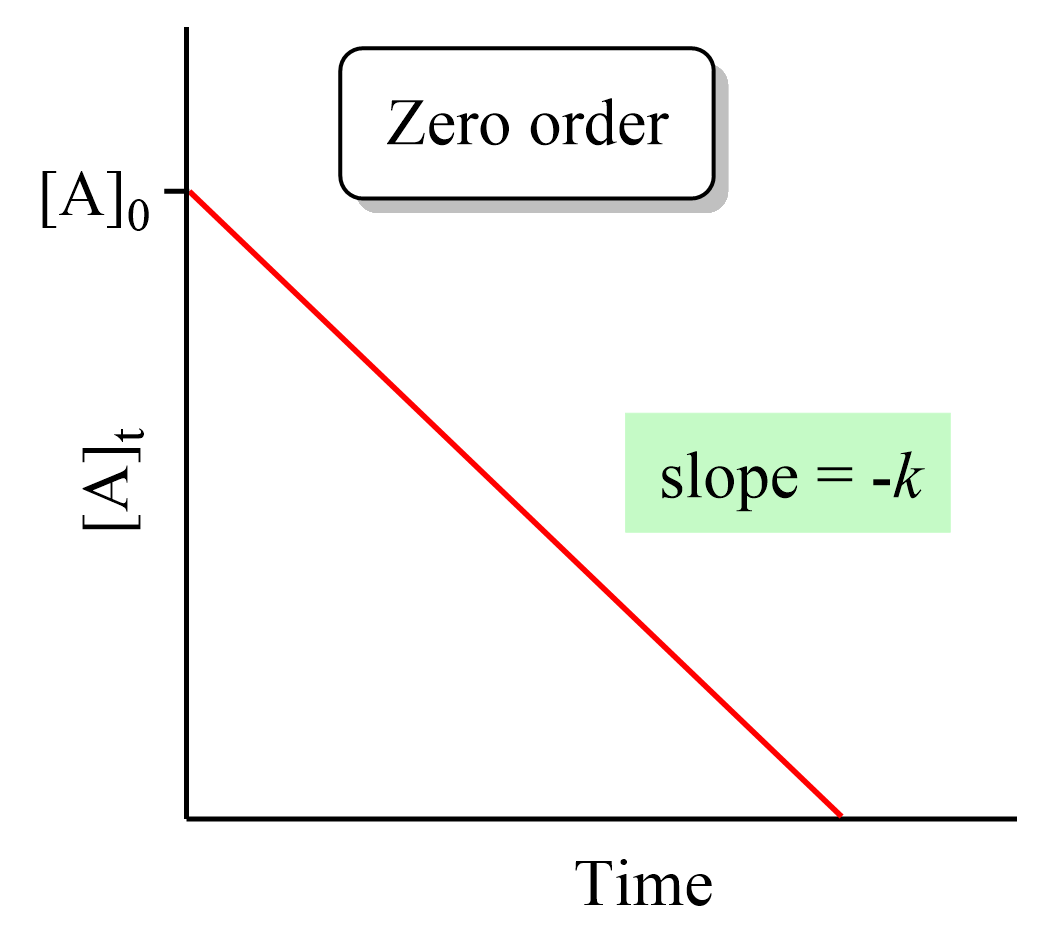
Zero Order Reactions Chemistry Steps
https://general.chemistrysteps.com/wp-content/uploads/2022/04/Zero-order-rate-law-liner-equation-graph.png
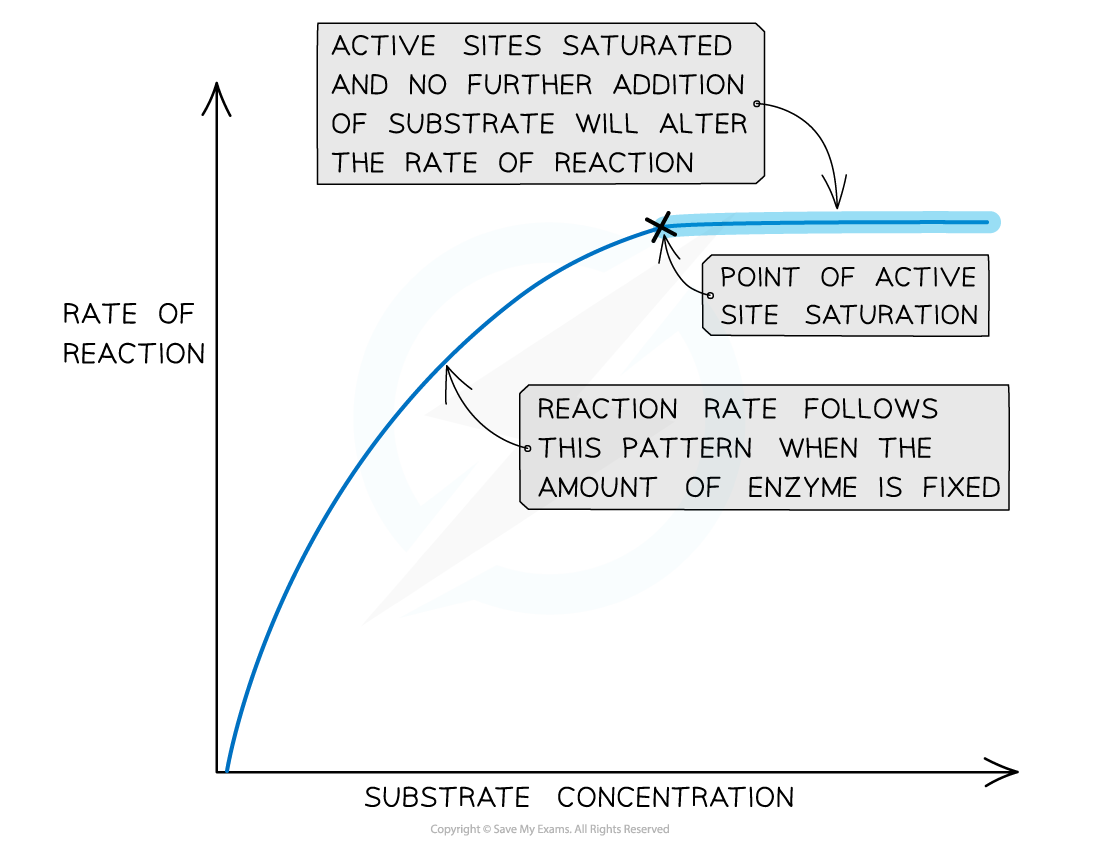
CIE A Level Biology 3 2 4 Rate Substrate Concentration
https://oss.linstitute.net/wechatimg/2022/08/The-effect-of-substrate-concentration-on-an-enzyme-catalysed-reaction-3.png
To determine the order of the reaction graphically we can plot the concentration of the reactants against the rate of the reaction If the slope of the line is proportional to the concentration of Apr 21 2023 nbsp 0183 32 The half life of any first order reaction does not depend upon the initial concentration of the reactant as t 1 2 0 693 k The concentration vs time graph of a first order
An easy test for a first order reaction is to plot the natural logarithm of a reactant concentration versus time and see if the graph is linear The data from Table 20 1 are plotted in Figure 20 4 and the rate constant k is derived from the slope of Jan 7 2025 nbsp 0183 32 Reaction Orders Graphs Reaction Order Using Concentration Time Graphs In a zero order reaction the concentration of the reactant is inversely proportional to time This
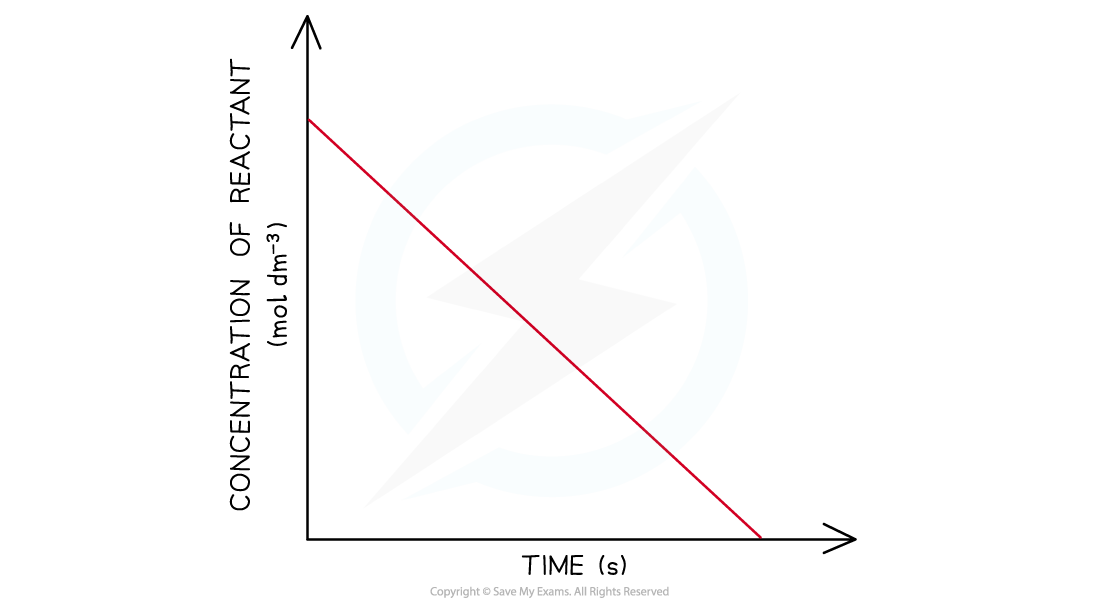
AQA A Level Chemistry 5 2 6 Concentration Time Graphs
https://oss.linstitute.net/wechatimg/2022/06/5.6-Reaction-Kinetics-Zero-Order-Concentration-3.png
https://chem.libretexts.org/@api/deki/files/15948/Eqn_14.21.jpg?revision=1
First Order Reaction Graph Between Concentration And Time - Concentration vs Time First Order Reaction This graph shows the plot of the natural logarithm of concentration versus time for a first order reaction The decomposition of a pollutant in water