Explain Polar And Nonpolar Molecules With Examples May 7 2020 nbsp 0183 32 Here are examples of polar and nonpolar molecules a look at how polarity relates to ionic and covalent bonds and how you can use polarity to predict which molecules will mix Nonpolar bonds form between two nonmetals with the same electronegativity value
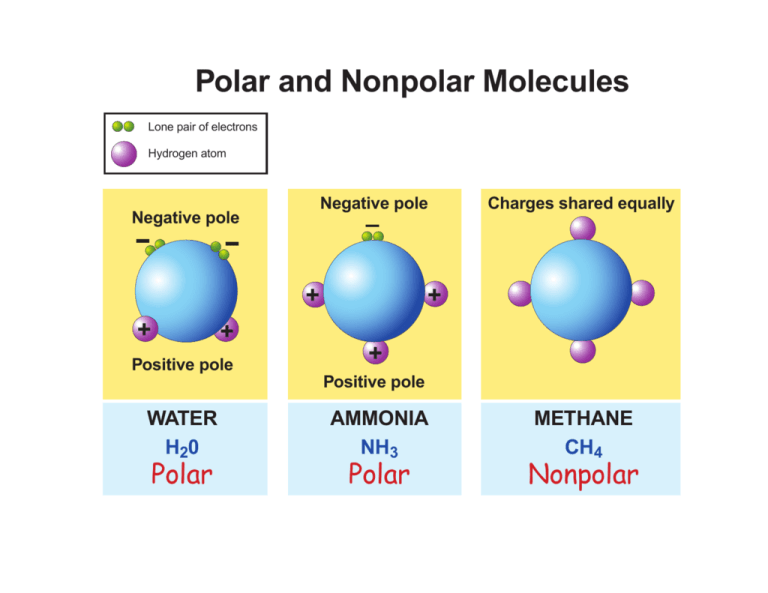
In this chemistry tutorial we explain the difference between polar bonds and non polar bonds We then tell you the definition of a polar molecule and what a non polar molecule is Last but not least you learn what a dipole moment is Aug 10 2022 nbsp 0183 32 Figure PageIndex 1 Some examples of nonpolar molecules based on molecular geometry BF 3 and CCl 4 Polar molecules are asymmetric either containing lone pairs of electrons on a central atom or having atoms with different electronegativities bonded
Explain Polar And Nonpolar Molecules With Examples
Explain Polar And Nonpolar Molecules With Examples
https://biologydictionary.net/wp-content/uploads/2017/02/Water-is-polar-molecule.jpg

Polar And Nonpolar Molecules
https://sciencenotes.org/wp-content/uploads/2020/05/NonPolarPolar-1024x683.png
Difference Between Polar And Nonpolar Molecules Definition Formation
http://pediaa.com/wp-content/uploads/2017/02/Difference-Between-Polar-and-Nonpolar-Molecules-3.jpg
Feb 3 2017 nbsp 0183 32 The main difference between polar and nonpolar molecules is net dipole moment The net dipole moment is formed on the atoms of polar molecules but not on non polar molecules This article explains 1 What are Polar Molecules Definition Formation Properties Examples 2 What are Nonpolar Molecules Apr 16 2023 nbsp 0183 32 We saw in the previous section that molecules can be classified as polar or nonpolar depending on the types of bonds present in the molecule and its overall molecular geometry The major difference between the two types of molecules was the presence of partially positive and partially negative regions in the polar molecule
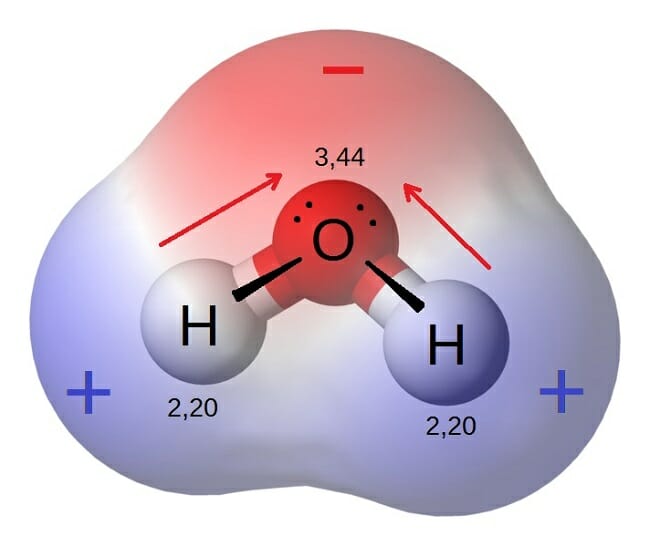
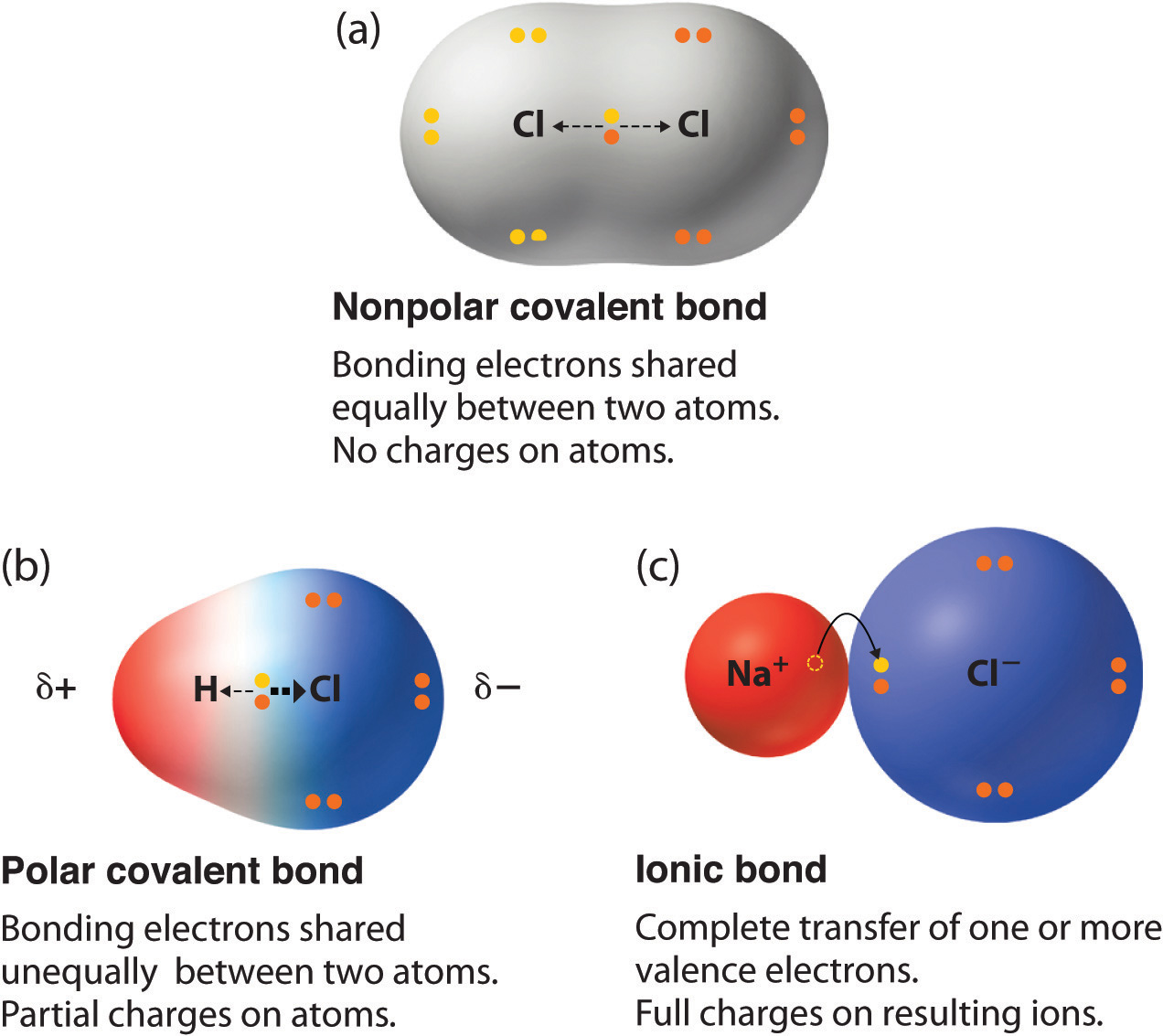
Mar 22 2021 nbsp 0183 32 Any covalent bond between atoms of different elements is a polar bond but the degree of polarity varies widely Some bonds between different elements are only minimally polar while others are strongly polar Ionic bonds can be considered the ultimate in polarity with electrons being transferred rather than shared Polar and non polar molecules A substance that contains polar covalent bonds may not be overall polar This is due to the shape of the molecule Water molecules are polar
More picture related to Explain Polar And Nonpolar Molecules With Examples
Polar And Nonpolar Molecules
https://s3.studylib.net/store/data/008336498_1-45c6da33a62230dfed17249c3e24c79d-768x994.png

Non Polar Molecules Examples Slideshare
https://thisonevsthatone.com/wp-content/uploads/Polar-vs-Nonpolar.jpg
Polar Covalent Bonds
https://2012books.lardbucket.org/books/principles-of-general-chemistry-v1.0/section_12/679fdbbd60e31f9fd4067f5f482a8f2c.jpg
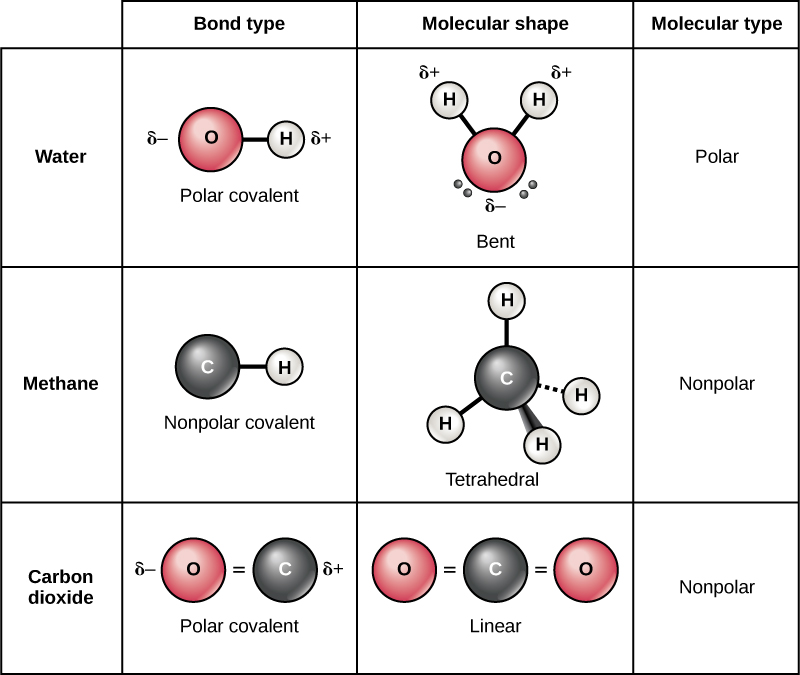
Explain how a molecule that contains polar bonds can be nonpolar Which of the following molecules contain polar bonds Which of these molecules and ions have dipole moments When a molecule contains more than one bond the geometry must be taken into account If the bonds in a molecule are arranged such that their bond moments cancel vector sum equals zero then the molecule is nonpolar This is the situation in CO 2 Figure PageIndex 14 Each of the bonds is polar but the molecule as a whole is nonpolar
Jan 20 2020 nbsp 0183 32 A polar molecule is a molecule containing polar bonds where the sum of all the bond s dipole moments is not zero Polar bonds form when there is a difference between the electronegativity values of the atoms participating in a bond In general pyramid shaped and V shaped molecules are said to be polar Whereas the Linear molecules are said to be non polar in nature Water is said to be a polar molecule due to the difference in the electronegativities between the oxygen atom and the hydrogen

Difference Between Polar And Nonpolar Molecules Definition Formation
http://pediaa.com/wp-content/uploads/2017/02/Difference-Between-Polar-and-Nonpolar-Molecules-Comparison-Summary.png
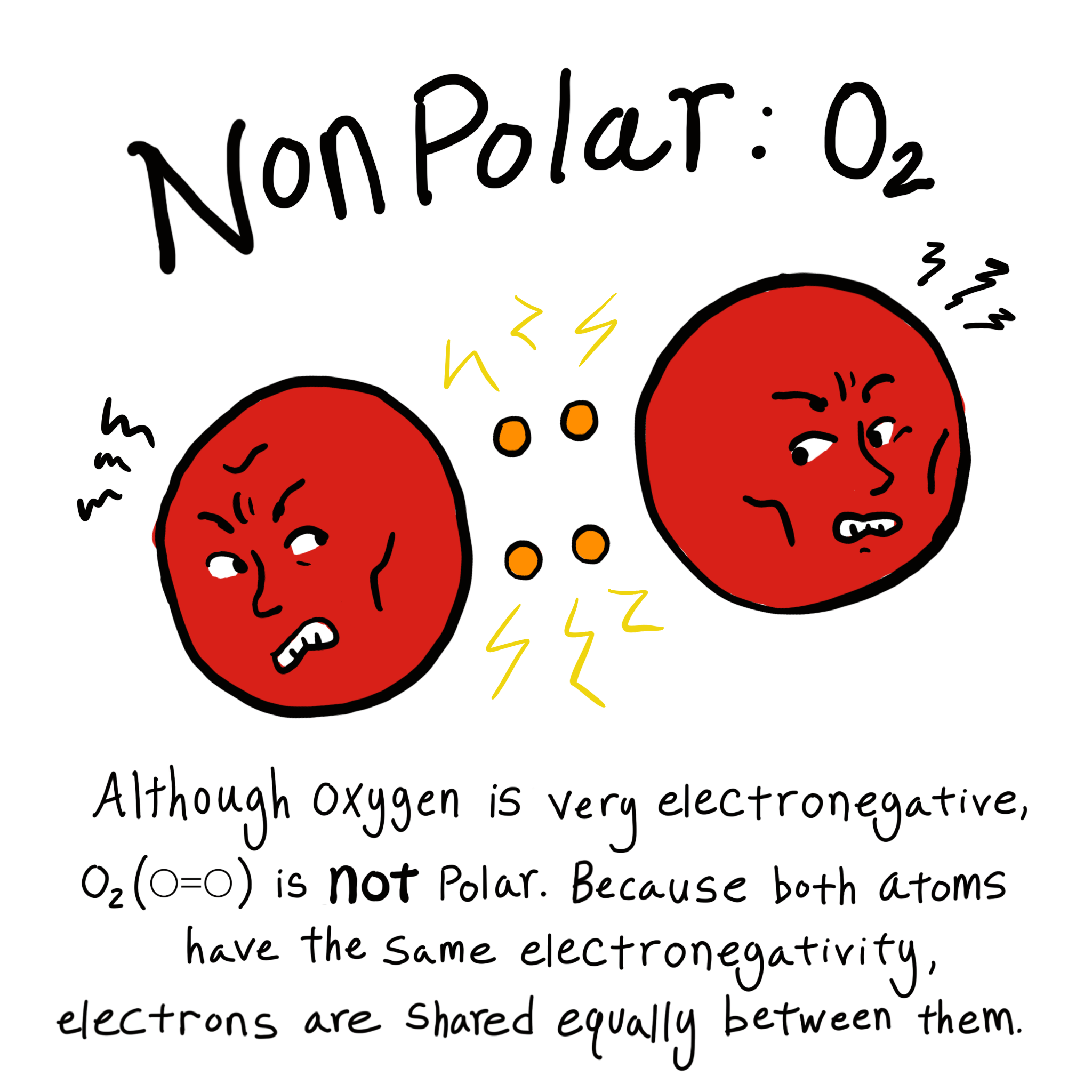
Polar Vs Nonpolar Bonds Overview Examples Expii
https://d20khd7ddkh5ls.cloudfront.net/oxygen_nonpolar_bond.png
Explain Polar And Nonpolar Molecules With Examples - Apr 16 2023 nbsp 0183 32 We saw in the previous section that molecules can be classified as polar or nonpolar depending on the types of bonds present in the molecule and its overall molecular geometry The major difference between the two types of molecules was the presence of partially positive and partially negative regions in the polar molecule