Empirical Formula Practice Worksheet Answer Key Answers to Worksheet 8 Empirical Formulas To calculate empirical formulas follow the steps outlined below assume percentages given in the problems are grams Step 1 convert to moles Step 2 divide each by the lowest number of moles Step 3 only if necessary multiply all by the same factor in order to obtain whole numbers
Empirical and Molecular Formula Worksheet SHOW WORK ON A SEPARATE SHEET OF PAPER Write the empirical formula for the following compounds 1 C6H6 2 C8H18 3 WO2 4 C2H6O2 5 X39Y13 6 A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole What is the molecular formula of this compound Empirical Formula Problems Set I 1 Determine the empirical formula of a compound consisting of a 55 3 K 14 6 P and 30 1 O K 3 PO 4 b 47 3 Cu and 52 7 Cl CuCl 2 c 52 14 C 13 13 H and 34 73 O C 2 H 6 O d 40 0 C 6 73 H and 53 3 O CH 2 O 2 In vanadium oxide the mole ratio is calculated to be 2 50 mol O 1 mol V
Empirical Formula Practice Worksheet Answer Key

Empirical Formula Practice Worksheet Answer Key
https://worksheets.clipart-library.com/images2/molecular-formula-worksheet-answers/molecular-formula-worksheet-answers-3.png
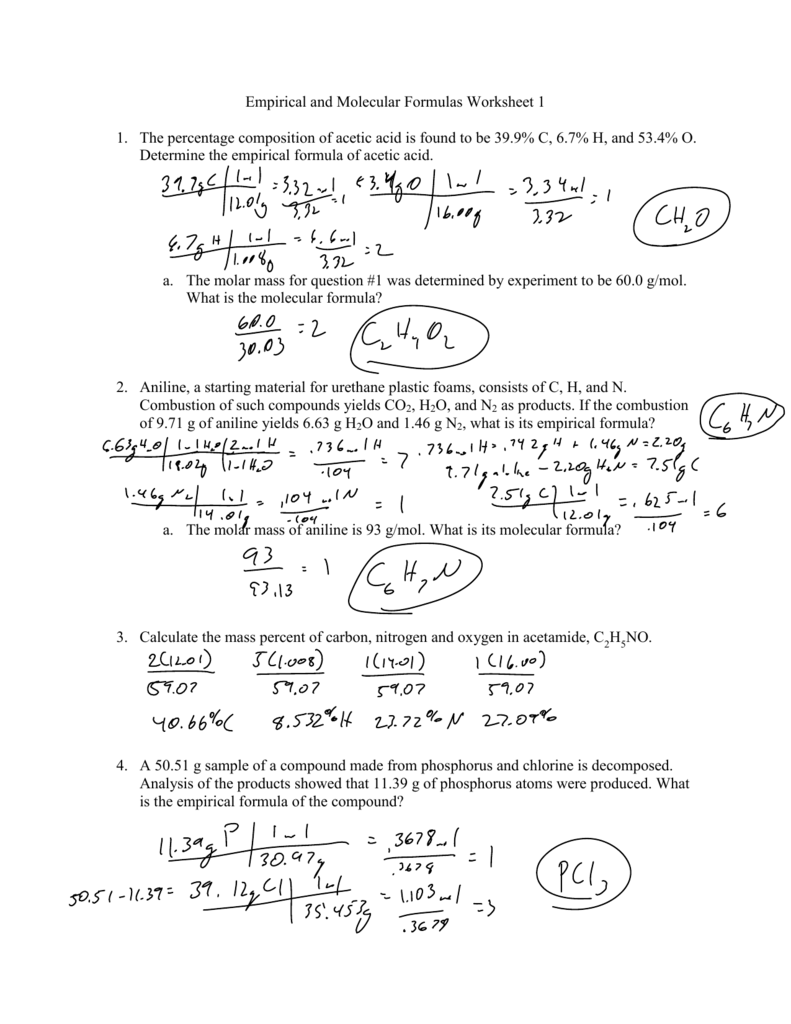
Empirical Formula Worksheets Key
https://www.unmisravle.com/wp-content/uploads/2018/04/empirical_and_molecular_formula_worksheet_2.png

Empirical And Molecular Formula Worksheets Answers
https://www.unmisravle.com/wp-content/uploads/2018/02/empirical_formula_worksheet_answers_free_worksheets_library_1.jpg
Jun 5 2021 nbsp 0183 32 View Empirical Formula practice worksheet answer key pdf from SNC 2D1 at Agincourt Collegiate Institute SCH3U Empirical and Molecular Formulas Practice worksheet Show all your work and the correct 5 D e t e rm i ne t he e m pi ri c a l a nd m ol e c ul a r form ul a of a c om pound c om pos e d of 18 24 g C a rbon 0 51 g H ydroge n a nd 16 91 g F l uori ne ha s a m ol a r m a s s 562 0 g m ol
Determine the empirical formula of lactic acid Determine the molecular formula Show your work for the calculation of the molecular formula here A compound is 21 20 Nitrogen 6 06 Hydrogen 24 30 Sulfur and 48 45 Oxygen Write the empirical formula for the compound Download Study notes Empirical and Molecular Formula Worksheet ANSWER KEY Brussels School of International Studies A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole
More picture related to Empirical Formula Practice Worksheet Answer Key

Determining Empirical Formulas Worksheet Answers Kayra Excel
https://i.pinimg.com/originals/a7/b4/50/a7b4503e28fb0ae680f1bf867069ef17.png

Empirical And Molecular Formula Practice By Teach Simple
https://teachsimplecom.s3.us-east-2.amazonaws.com/images/empirical-and-molecular-formula-practice/image-1653688677256-2.jpg

Determining Empirical Formulas Worksheets
https://db-excel.com/wp-content/uploads/2019/09/empirical-and-molecular-formulas-worksheet-1-1-the-percentage-749x970.png
Let the unknown metal be X As it is in group 1 the empirical formula must be XCl There is 47 6 Cl so the percentage composition of X must be 52 4 X 39 02 and the formula is KCl Pearson Education Australia a division of Pearson Australia Group Pty Ltd 2008 Empirical Formulas Worksheet 1 Directions Find the empirical formula and name for each of the following 1 A compound is 24 7 Calcium 1 2 Hydrogen 14 8 Carbon and 59 3 Oxygen Write the empirical formula and name the compound 2 A compound is 21 20 Nitrogen 6 06 Hydrogen 24 30 Sulfur and 48 45 Oxygen
Composition Empirical Formula and Molecular Formula Worksheet 1 A sample of an unknown compound with a mass of 0 g has the following composition 50 fluorine and 49 iron When this compound is decomposed into its elements what What is an empirical formula How is it different from a molecular formula The empirical formula is the lowest whole number ratio of atoms in a compound The molecular formula shows the true number of atoms in the molecule and is a whole number multiple of the empirical formula State the empirical formula for each of the following a C 6 H

Ch 10 Empirical And Molecular Formula Worksheet
https://s3.studylib.net/store/data/025187267_1-c545c8bf7e045873fff8372e23497e22.png

Empirical Formula Practice Worksheet
https://i.ytimg.com/vi/EqPWhjO0bu4/maxresdefault.jpg
Empirical Formula Practice Worksheet Answer Key - 5 D e t e rm i ne t he e m pi ri c a l a nd m ol e c ul a r form ul a of a c om pound c om pos e d of 18 24 g C a rbon 0 51 g H ydroge n a nd 16 91 g F l uori ne ha s a m ol a r m a s s 562 0 g m ol