Electronic Configuration Of Nitrogen This is the number of protons in the nuclei of nitrogen atoms A neutral atom has the same number of electrons as protons So the electron configuration will include 7 electrons
Due to this the elements can either lose five electrons or gain three electrons in order to attain the stable configuration The general electronic configuration of the nitrogen family is ns 2 np 3 Therefore the electronic configuration of sulfur can be written as 1s 2 2s 2 2p 6 3s 2 3p 4 The electronic configuration of elements can also be written with the help of noble gases These
Electronic Configuration Of Nitrogen

Electronic Configuration Of Nitrogen
https://www.shutterstock.com/shutterstock/photos/1669204432/display_1500/stock-photo-electronic-configuration-of-nitrogen-atom-energy-level-diagram-1669204432.jpg

168 Electronic Configuration Of Nitrogen Images Stock Photos Vectors
https://www.shutterstock.com/shutterstock/photos/2183588503/display_1500/stock-vector-nitrogen-atom-model-chemical-element-with-symbol-n-and-with-atomic-number-bohr-model-of-2183588503.jpg

168 Electronic Configuration Of Nitrogen Images Stock Photos Vectors
https://www.shutterstock.com/shutterstock/photos/1932619664/display_1500/stock-vector-bohr-model-of-the-nitrogen-atom-electron-structure-of-nitrogen-1932619664.jpg
Nitrogen molecule N 2 The electronic configuration of nitrogen Z 7 in ground state is 1 s 2 2 s 2 2 p 1 x 2 p 1 y 2 p 1 z Therefore the total number of electrons present in nitrogen molecule c The electronic configuration of nitrogen is 2 5 How many electrons in the outer shell of a nitrogen atom are not involved in the formation of a nitrogen molecule d In the formation of
If the nitrogen atom had electronic configuration 1 S 7 it would have enrgy lower than that of the normal ground state configuration 1 s 2 2 s 2 2 p 3 because the electrons would be closer to If the nitrogen atom had an electronic configuration of 1 s 7 it would have lower energy than that of the normal ground state configuration 1 s 2 2 s 2 2 p 3 because the electrons would be closer
More picture related to Electronic Configuration Of Nitrogen

How To Write The Electronic Configuration Of Nitrogen Chemical
https://i.ytimg.com/vi/9E1Al9Hj5cE/maxresdefault.jpg

Nitrogen Electronic Configuration how To Write Nitrogen Electronic
https://i.ytimg.com/vi/NZl36Klaku4/maxresdefault.jpg

Electronic Configuration For Nitrogen Spdf Trick Chemistry
https://i.ytimg.com/vi/Vrg0qFYKpIM/maxresdefault.jpg
Ground state electronic configuration of nitrogen atom can be represented by 1 4 only All the unpaired If the nitrogen atom had electronic configuration 1 S 7 it would have enrgy lower than that of the normal ground state configuration 1 s 2 2 s 2 2 p 3 because the electrons would be closer to
[desc-10] [desc-11]

1 648 Number Electrons Energy Level Images Stock Photos Vectors
https://www.shutterstock.com/shutterstock/photos/1914333358/display_1500/stock-vector-nitrogen-atomic-structure-has-atomic-number-atomic-mass-electron-configuration-and-energy-levels-1914333358.jpg
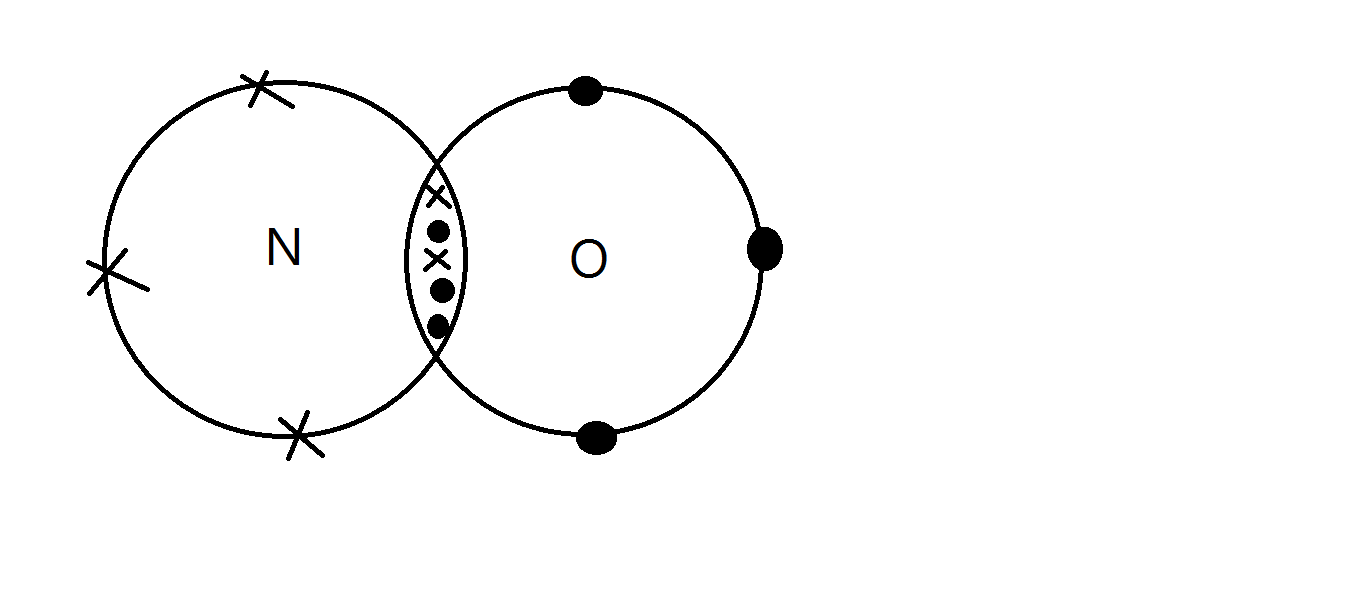
2P3 LSS 2P333 Nitric Oxide
https://2.bp.blogspot.com/-j0Ebk_6UIuw/TVZf6MPG9FI/AAAAAAAAACQ/guFYCPZAMVA/s1600/nitric+oxygen.png
Electronic Configuration Of Nitrogen - If the nitrogen atom had an electronic configuration of 1 s 7 it would have lower energy than that of the normal ground state configuration 1 s 2 2 s 2 2 p 3 because the electrons would be closer