Electron Dot Diagram Practice Worksheet Answers Answer the following questions and check your answers below These problems are for practice only will not be graded Be sure you know how to draw correct Lewis Dot Structures and are able to correctly predict the electronic arrangement and molecular geometry before going on to
How To write an electron configuration Determine the total number of electrons to be represented Use the Aufbau process to fill the orbitals with electrons The Aufbau process requires that electrons fill the lowest energy orbitals first In another words atoms are built from the ground upwards Practice Sheet Electron Dot Lewis Structures A Lewis or Electron Dot Structure is a convenient representation of the valence electrons in an atom An electron dot structure for an atom is simply the symbol for the element surrounded by a number of dots equal to the number of valence electrons
Electron Dot Diagram Practice Worksheet Answers
Electron Dot Diagram Practice Worksheet Answers
https://imgv2-1-f.scribdassets.com/img/document/360479464/original/76f49666cd/1516979920?v=1

Which Atom Is Which Worksheet
https://i.pinimg.com/originals/df/6f/48/df6f480145c60e09b11105ac1e5d6d4a.jpg

Lewis Dot Diagrams Chem Worksheet 5 7
https://i.pinimg.com/originals/92/5e/43/925e43a3d971b785ae57d8be6bc5475a.jpg
Answer Each bond includes a sharing of electrons between atoms Two electrons are shared in a single bond four electrons are shared in a double bond and six electrons are shared in a triple bond Electron Dot Structure or Lewis Dot Diagram Gilbert Lewis A notation showing the valence electrons surrounding the atomic symbol column your element is in This will tell you the number of valence electrons your element has You will only draw the valence electrons Write the element symbol
Draw the electron dot diagrams for all of the atoms in the molecule Draw the atom with the highest bonding capacity in the this is usually the atom in the chemical formula Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons Once you have found the number of valance electrons place them around the elements symbol Making Ions Remember that atoms want
More picture related to Electron Dot Diagram Practice Worksheet Answers

Lewis Dot Diagram Practice
https://s2.studylib.net/store/data/010001948_1-d42cec3a405cb0abadea501d80316357.png

Lewis Electron Dot Diagram Worksheet
https://www.worksheeto.com/postpic/2010/12/lewis-dot-structure-practice-worksheet_427095.png
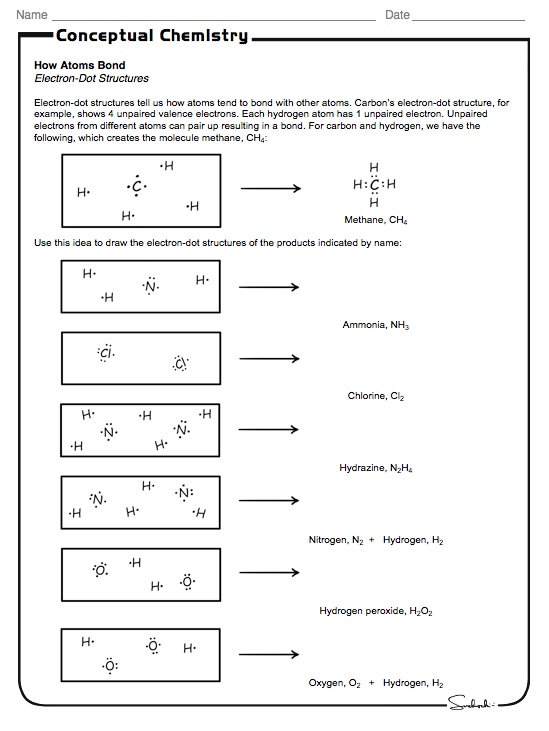
15 1 Electron Dot Structures Conceptual Academy
http://styraki.com/files/CCElectronDot.jpg
Showing 8 worksheets for Electron Dot Structure Worksheets are 5 11 electron diagrams and lewis structures wkst Lewis dot structures and molecule ge How to Draw a Lewis Dot Structure Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion You can quickly refer to the periodic table for the group A number for this information
Aug 13 2021 nbsp 0183 32 Steps to determine the electron dot diagram 1 Analyse the total number of valence electrons of every atom in a molecule 2 In case of anion molecule add the extra electrons around the element while drawing dot diagram Valence Electrons and Lewis Dot Structure Worksheet Answers Free download as PDF File pdf or read online for free
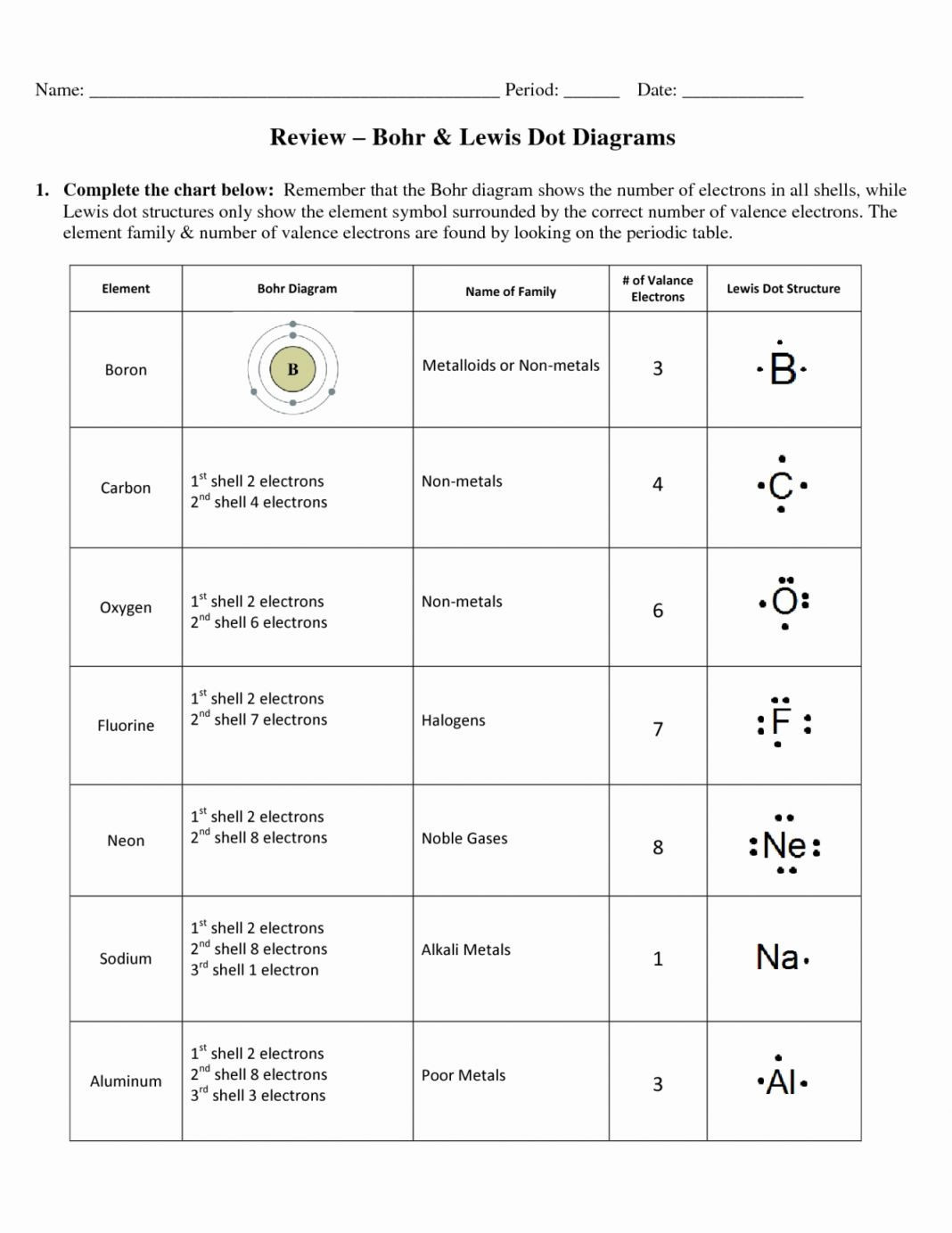
Bohr Model Blank And Lewis Dot Diagram Worksheet Answers Db excel
https://db-excel.com/wp-content/uploads/2019/09/bohr-model-blank-and-lewis-dot-diagram-worksheet-answers.jpg
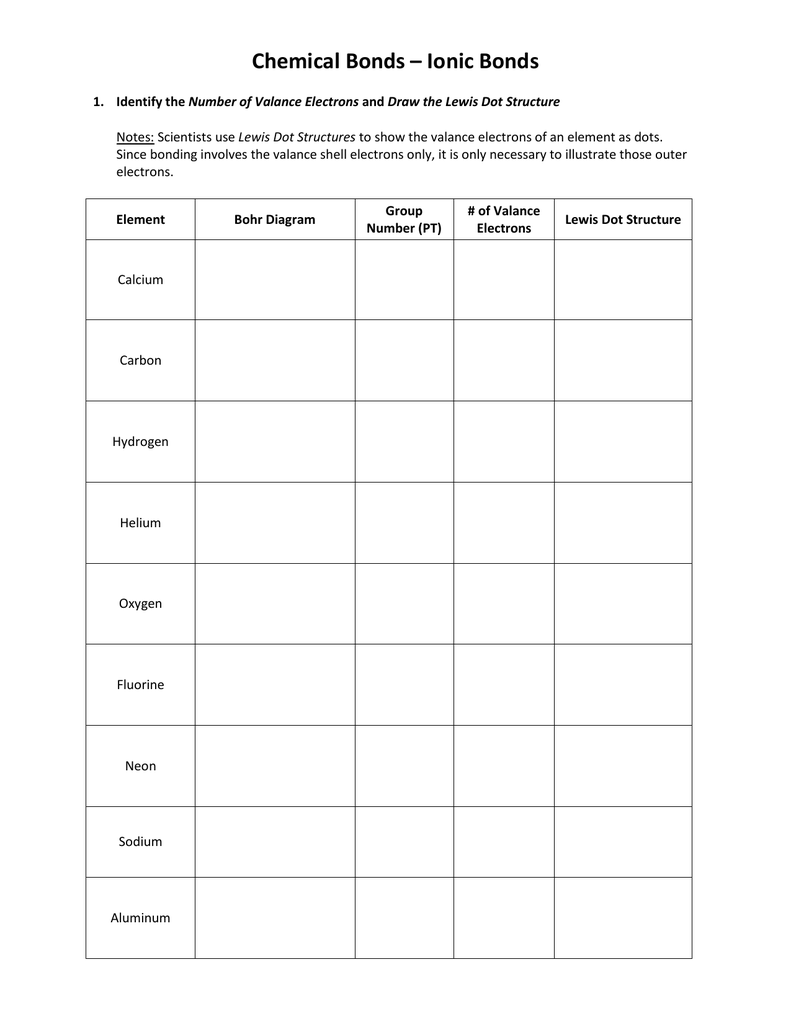
Lewis Dot Structures Worksheet
https://s2.studylib.net/store/data/010212602_1-7d40b2312f1ea3c3e061ca00f159c411.png
Electron Dot Diagram Practice Worksheet Answers - Draw the electron dot diagrams for all of the atoms in the molecule Draw the atom with the highest bonding capacity in the this is usually the atom in the chemical formula
