Drug Approval Process In India Slideshare About WHO Drug Information WHO Drug Information is a quarterly journal providing an overview of topics relating to medicines development and regulation which is targeted to a wide
Drug 152 Drug Jun 25 2024 nbsp 0183 32 A new report from the World Health Organization WHO highlights that 2 6 million deaths per year were attributable to alcohol consumption accounting for 4 7 of all deaths
Drug Approval Process In India Slideshare

Drug Approval Process In India Slideshare
https://i.ytimg.com/vi/eIeAGpLNy7w/maxresdefault.jpg

New Drug Approval Process In India I Hindi YouTube
https://i.ytimg.com/vi/S9_htt2GCQM/maxresdefault.jpg

Drug Approval Process In India YouTube
https://i.ytimg.com/vi/jYZzeEhziN8/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AG-B4AC0AWKAgwIABABGGUgZShlMA8=&rs=AOn4CLChCBC3E9snJb-Jhmo_CjGDp1ZpTw
Jun 20 2025 nbsp 0183 32 Overview Drug addiction also called substance use disorder is a disease that affects a person s brain and behavior and leads to an inability to control the use of a legal or Look up information about prescription drugs over the counter medications herbs vitamins and supplements
May 17 2024 nbsp 0183 32 The World Health Organization WHO today released its updated Bacterial Priority Pathogens List BPPL 2024 featuring 15 families of antibiotic resistant bacteria In the Anatomical Therapeutic Chemical ATC classification system the active substances are divided into different groups according to the organ or system on which they act and their
More picture related to Drug Approval Process In India Slideshare

Regulatory Requirements And Drug Approval Process In India BP 702T
https://i.ytimg.com/vi/xkAep-G53Cc/maxresdefault.jpg

Biosimilars Novum
https://www.novumprs.com/wp-content/uploads/2022/05/biosimilar-approval-pathway.jpg
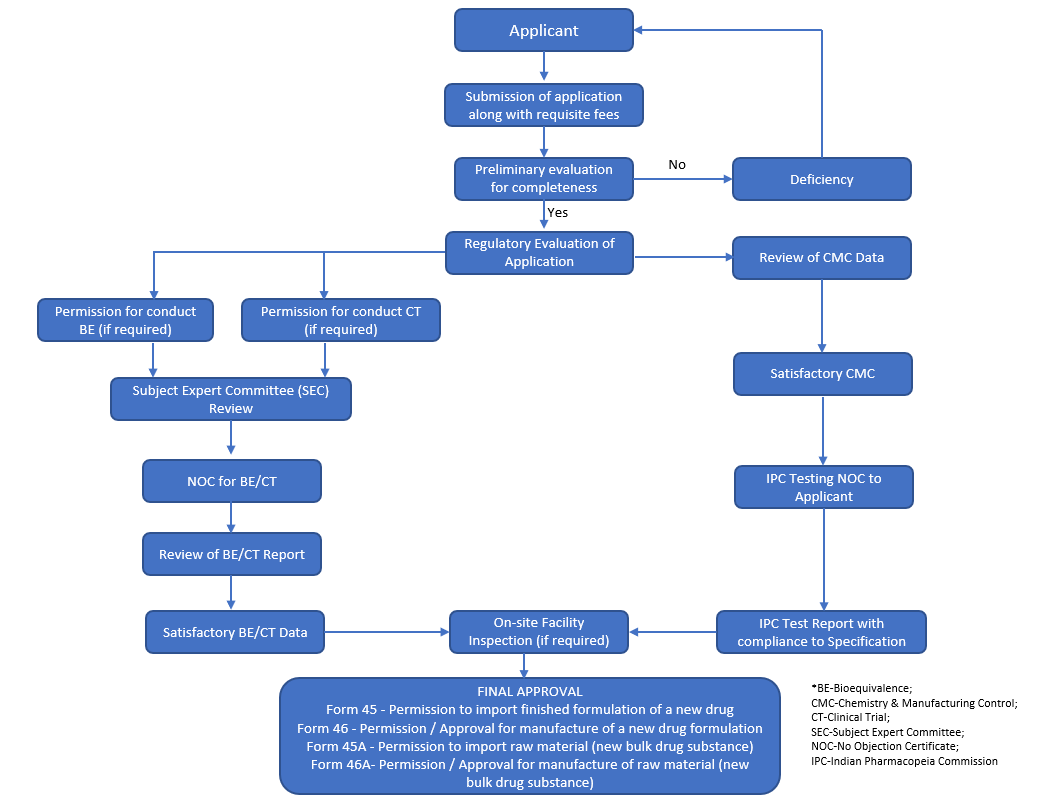
India Regweb
https://regweb.ca/wp-content/uploads/2021/10/India.png
The Unit works globally to improve health and well being of populations by articulating promoting supporting and monitoring evidence informed policies strategies and interventions to reduce Other patterns of drug resistance There were an estimated 1 4 million incident cases 95 UI 0 62 2 1 million of isoniazid resistant TB in 2023 including people with both rifampicin
[desc-10] [desc-11]

Generic Drug Approval Process In INDIA 12 Download Scientific Diagram
https://www.researchgate.net/profile/Satbir_Singh20/publication/325145465/figure/fig2/AS:626451065872387@1526368981583/Generic-drug-approval-process-in-INDIA-12.jpg
Generic Drug Approval Process Download Scientific Diagram
https://www.researchgate.net/profile/Brahmaiah_Bonthagarala/publication/311986176/figure/fig2/AS:445278504394753@1483174076189/Generic-drug-approval-process.ppm
Drug Approval Process In India Slideshare - [desc-13]