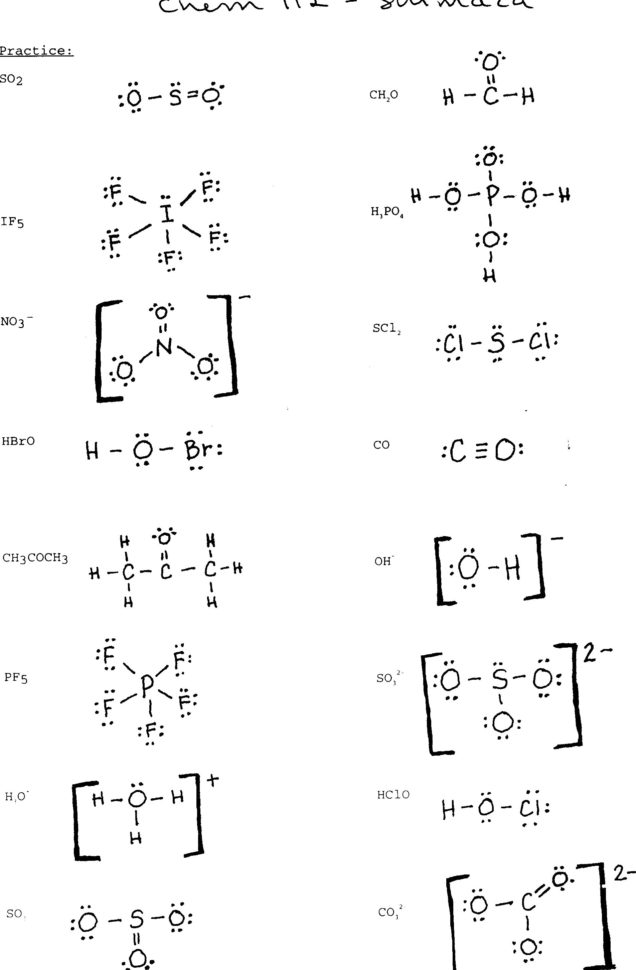
Drawing Lewis Structures Worksheet Pdf Draw the Lewis Structures for each of the following molecules If you are not sure if your structure is correct do a formal charge check You should consult the Lewis structure rules and a periodic table while doing this exercise
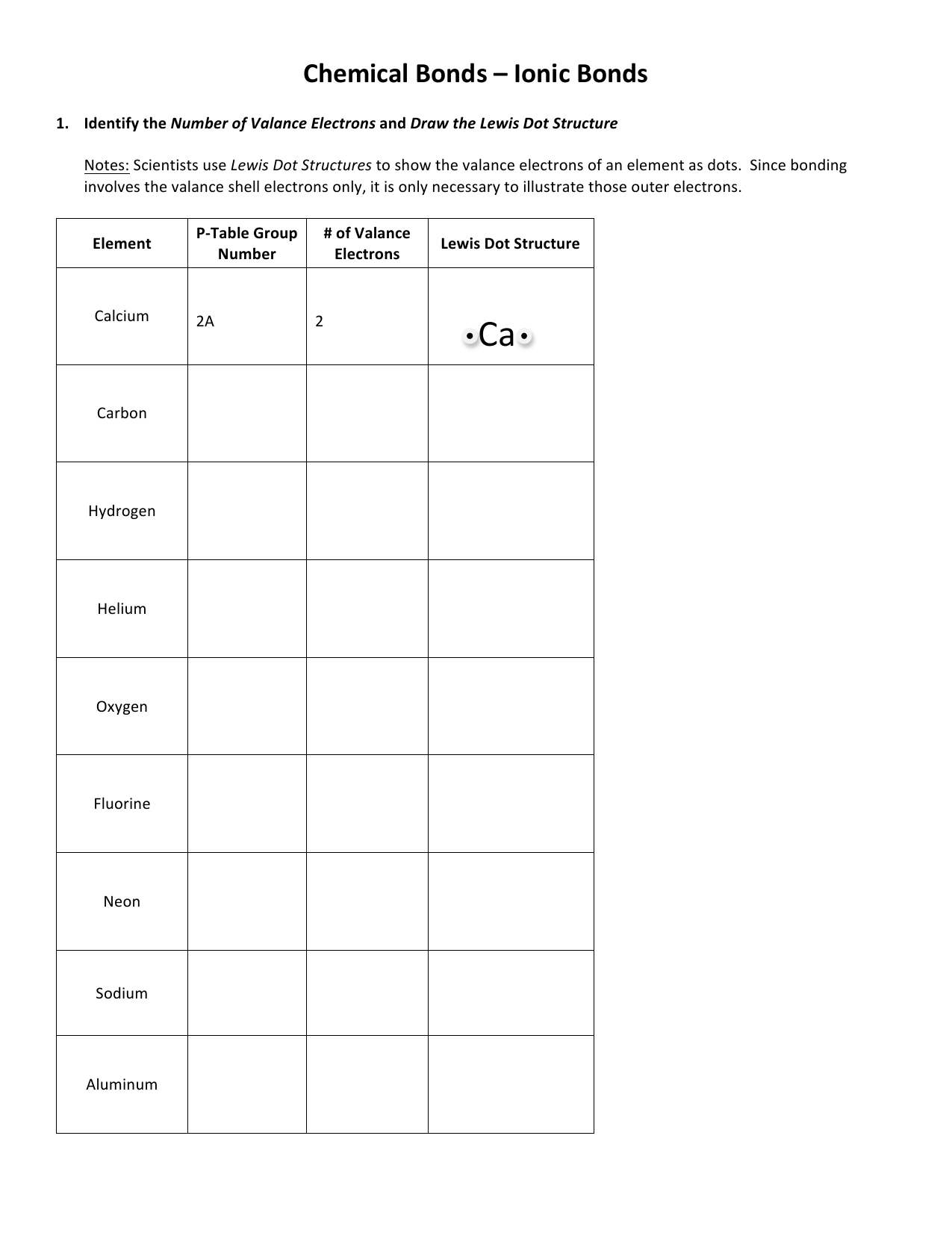
For the following polyatomic ions write the ion s name calculate the number of total valence electrons being sure to take the charge into account and draw the correct Lewis structures Remember for polyatomic ions the normal number of covalent bonds may not always apply This worksheet includes some rules and guidelines to help you draw Lewis structures Identify the central atom of the molecule This is the least electronegative atom See back of periodic table for values or the only one able to form more than one bond
Drawing Lewis Structures Worksheet Pdf

Drawing Lewis Structures Worksheet Pdf
https://i.pinimg.com/originals/af/6b/e6/af6be6f54bce58f1aa0a0d30940620df.jpg

Lewis Structure Practice Worksheet Zipworksheet
https://s3.studylib.net/store/data/008472361_1-9ce0d8f0f67db63b2bd132e4a5b3d200.png

Lewis Structures Practice Worksheet Lewis Dot Structure Easy Hard
https://i1.wp.com/3.files.edl.io/1641/18/10/24/161346-e6d89c5e-a837-4293-8005-6d7c5ba30e9f.jpg
Draw the Lewis Structures for each of the following molecules If you are not sure if your structure is correct do a formal charge check You should consult the Lewis structure rules and a periodic table while doing this exercise Read the Instructions for Drawing Lewis Structures worksheet carefully and complete Lewis structures for each of the following molecules Group A Simple Molecules CH
Worksheet on Lewis Structures 1 LewisStructuresHwrk odt Lewis Structures Homework Draw the Lewis structures for the following compounds Make certain that a All structures follow the octet rule b There are the correct number of valence electrons c All atoms have their correct charge 1 PI3 2 N2 3 H2O 4 AsBr3 5 SiCl4 Lewis Dot Structures Practice Sheet page 3 Steps for Drawing Lewis Dot Structures for Larger Molecules 1 First determine the central atom a Hydrogens H and halogens F Cl Br I are almost always outer atoms They only want to form one bond to get to a noble gas con guration
More picture related to Drawing Lewis Structures Worksheet Pdf

Lewis Structure Worksheet 1
https://i.pinimg.com/736x/1b/bd/4e/1bbd4e5f9dca2ffe5687fe09a6ba8823.jpg
Lewis Dot Structure Practice Worksheet Db excel
https://db-excel.com/wp-content/uploads/2019/09/lewis-structures-worksheet-video-with-answers-youtube-lewis-636x970.jpg

Lewis Dot Structures Worksheet 1 Answer Key Free Worksheets Samples
https://www.housview.com/wp-content/uploads/2018/12/lewis_structure_practice_worksheet_11_4.jpg
Read the Instructions for Drawing Lewis Structures worksheet carefully and complete Lewis structures for each of the following molecules Remember the first atom in the name that isn t H is the central atom where applicable Draw Lewis structures for the following Include any resonance structures If more than one Lewis structure can be drawn use formal charges to decide on the most preferred Lewis structure Answers to these will be posted on the web late Friday afternoon Also included is a blank for Molecular Geometry
Drawing the molecule Look up the electronegativity values for each element in your structure The least electronegative atom represents the central atom Hydrogen is the only exception to this since it forms only one bond Arrange the remaining atoms symmetrically around the central atom Using the concept of a central atom bonded to two or more terminal atoms draw a skeleton structure joining the atoms by single bonds Count how many single bonds are present

Drawing Lewis Structures Practice Worksheet Warehouse Of Ideas
https://i.pinimg.com/originals/6e/c5/ee/6ec5ee1358d95a893f868c0f8b97bc7d.jpg
Drawing Lewis Structures Worksheet Abhayjere
https://s3.studylib.net/store/data/025209243_1-3edaa89b29b8ecfc126df27949fbf52b.png
Drawing Lewis Structures Worksheet Pdf - Draw Lewis structures for the following 6 PBr 3 7 N 2 H 2 8 CH 3 OH 9 NO 2 1 10 C 2 H 4 11 Write the Lewis dot structure for each of these molecules Some are easy some are not If you get really stuck skip it and move onto the next one Come back to it later or ask for help