Does Molecular Geometry Affect Polarity The polarity of a bond the extent to which it is polar is determined largely by the relative electronegativities of the bonded atoms Electronegativity chi was defined as the ability of an atom in a molecule or an ion to attract electrons to
Sep 16 2014 nbsp 0183 32 Determine the polarity of molecules using net molecular dipoles Molecules have shapes There is an abundance of experimental evidence to that effect from their Determine the polarity of molecules using net molecular dipoles Molecules have shapes There is an abundance of experimental evidence to that effect from their physical properties to their chemical reactivity
Does Molecular Geometry Affect Polarity
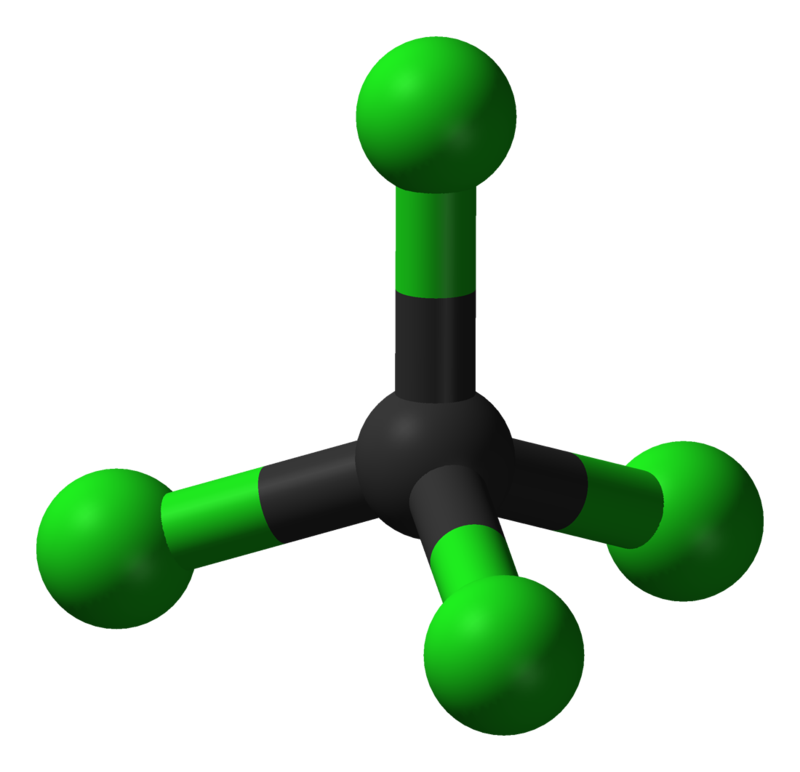
Does Molecular Geometry Affect Polarity
https://pediaa.com/wp-content/uploads/2017/02/How-Does-Molecular-Shape-Affect-Polarity-6.png
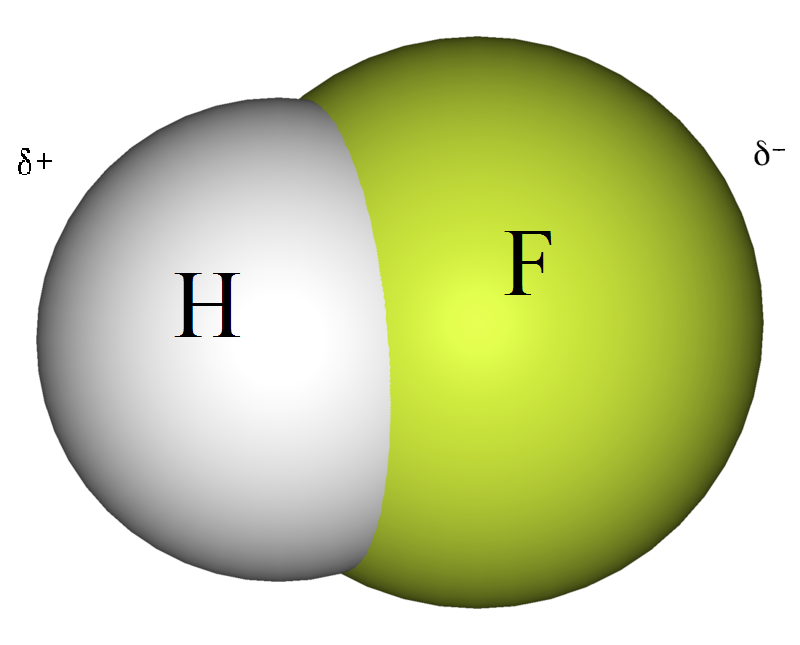
How Does Molecular Shape Affect Polarity Explained With Examples
https://pediaa.com/wp-content/uploads/2017/02/How-Does-Molecular-Shape-Affect-Polarity-2.png

How Does Molecular Geometry Affect Polarity
http://chem.libretexts.org/@api/deki/files/43913/03a57d057652402545c1e8b4fd91332b_-_Copy.jpg?revision=1&size=bestfit&height=275
How Does Molecular Shape Affect Physical Properties The shape of a molecule has an effect on its polarity which can be either positive or negative Polar compounds dissolve in polar solutions and have higher boiling points For a molecule the overall dipole moment is determined by both the individual bond moments and how these dipoles are arranged in the molecular structure Polar molecules those with
Jul 16 2020 nbsp 0183 32 Properties of Polar Molecules Polar molecules tend to align when placed in an electric field with the positive end of the molecule oriented toward the negative plate and the negative end toward the positive plate Determine the Lewis structure molecular geometry and polarity for molecules using Lewis structures Sketch molecular geometries on paper with wedges and dashes to represent their 3D shape Explain how bonding affects the ability
More picture related to Does Molecular Geometry Affect Polarity
Solved Lab 10 Molecular Geometry Lab Lewis Structure Molecular
https://www.coursehero.com/qa/attachment/37199081/

Is SF2 Polar Or Nonpolar Techiescientist
https://techiescientist.com/wp-content/uploads/2020/09/Bent-3D-balls.png

Lab 7 Report FORM Spring 2023 Lab 7 How Does Molecular Shape Affect
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/c3f0322c40741544ce1807a741c31282/thumb_1200_1553.png
Thus the electron pair geometry is tetrahedral and the molecular structure is bent with an angle slightly less than 109 5 176 In fact the bond angle is 104 5 176 Figure 15 10 10 a H 2 O has four For molecules of high symmetry such as latex ce BF3 latex trigonal planar latex ce CH4 latex tetrahedral latex ce PF5 latex trigonal bipyramidal and latex ce SF6 latex octahedral all the bonds are of identical polarity
Two regions of electron density around a central atom in a molecule form a linear geometry three regions form a trigonal planar geometry four regions form a tetrahedral geometry five Describe how molecular geometry plays a role in determining whether a molecule is polar or nonpolar Distinguish between the following three types of intermolecular forces dipole dipole

Lab 6 Report FORM F22 Lab 6 How Does Molecular Shape Affect Polarity
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/77f958f37f5bacbf18f133a165de564f/thumb_1200_1553.png

VSEPR Theory Molecular Geometry AP Chem Unit 2 Topic 7B YouTube
https://i.ytimg.com/vi/a8xDFQLYtYE/maxresdefault.jpg
Does Molecular Geometry Affect Polarity - Determine the Lewis structure molecular geometry and polarity for molecules using Lewis structures Sketch molecular geometries on paper with wedges and dashes to represent their 3D shape Explain how bonding affects the ability