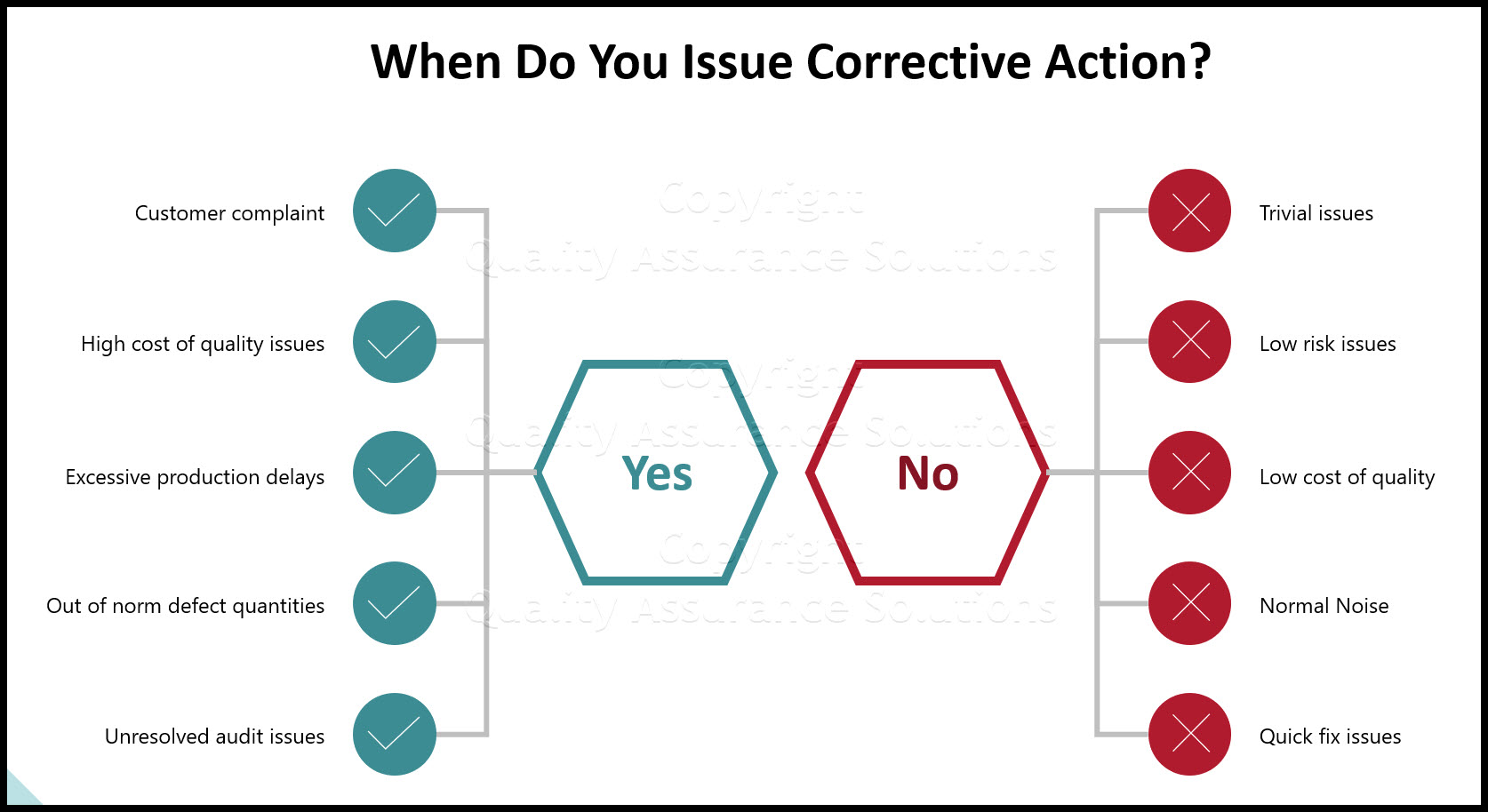
Corrective Action Plan Steps The Therapeutic Goods Act 1989 prohibits import into or supply in Australia of unapproved therapeutic goods for use in humans unless they are included in the ARTG or otherwise the
Feb 6 2025 nbsp 0183 32 Unapproved therapeutic goods are products not listed on the Australian Register of Therapeutic Goods ARTG and are generally prohibited from import and sale in Australia Jan 29 2024 nbsp 0183 32 The following avenues provide for the importation into or supply in Australia of unapproved therapeutic goods for use in a clinical trial Clinical Trial Notification CTN
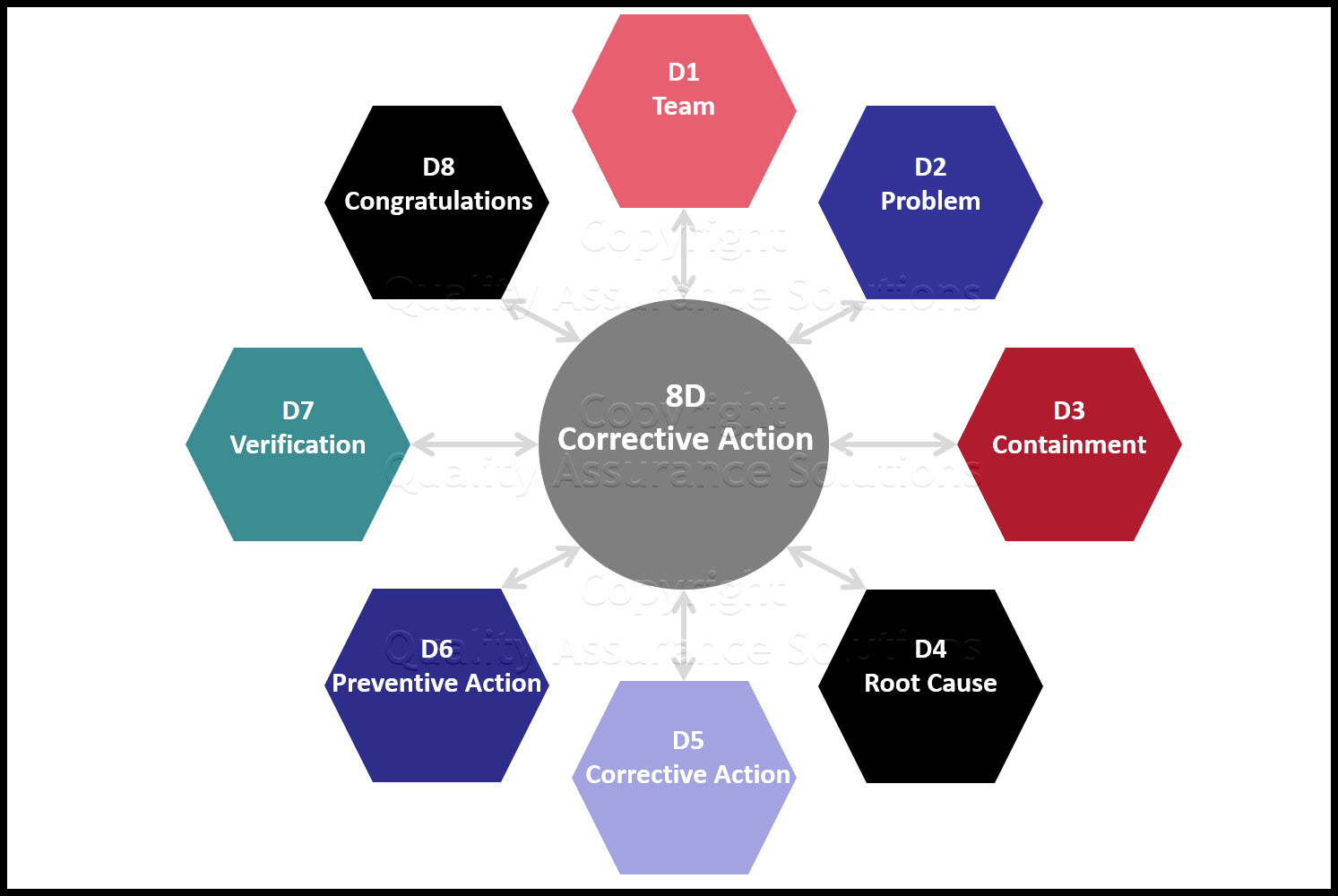
Corrective Action Plan Steps

Corrective Action Plan Steps
https://www.collidu.com/media/catalog/product/img/2/8/28d29cf8250143dd6f7d21d8948f918083829e431c804c683520c50c0b4fdd4e/corrective-action-slide3.png
![]()
Corrective Action Tracking Template
https://www.eloquens.com/i/p/3/3846/58838/1/health-safety-corrective-and-preventive-action-tracker.png
Desigual Return Form D1 D2 D3 Hotsell Innoem eng psu ac th
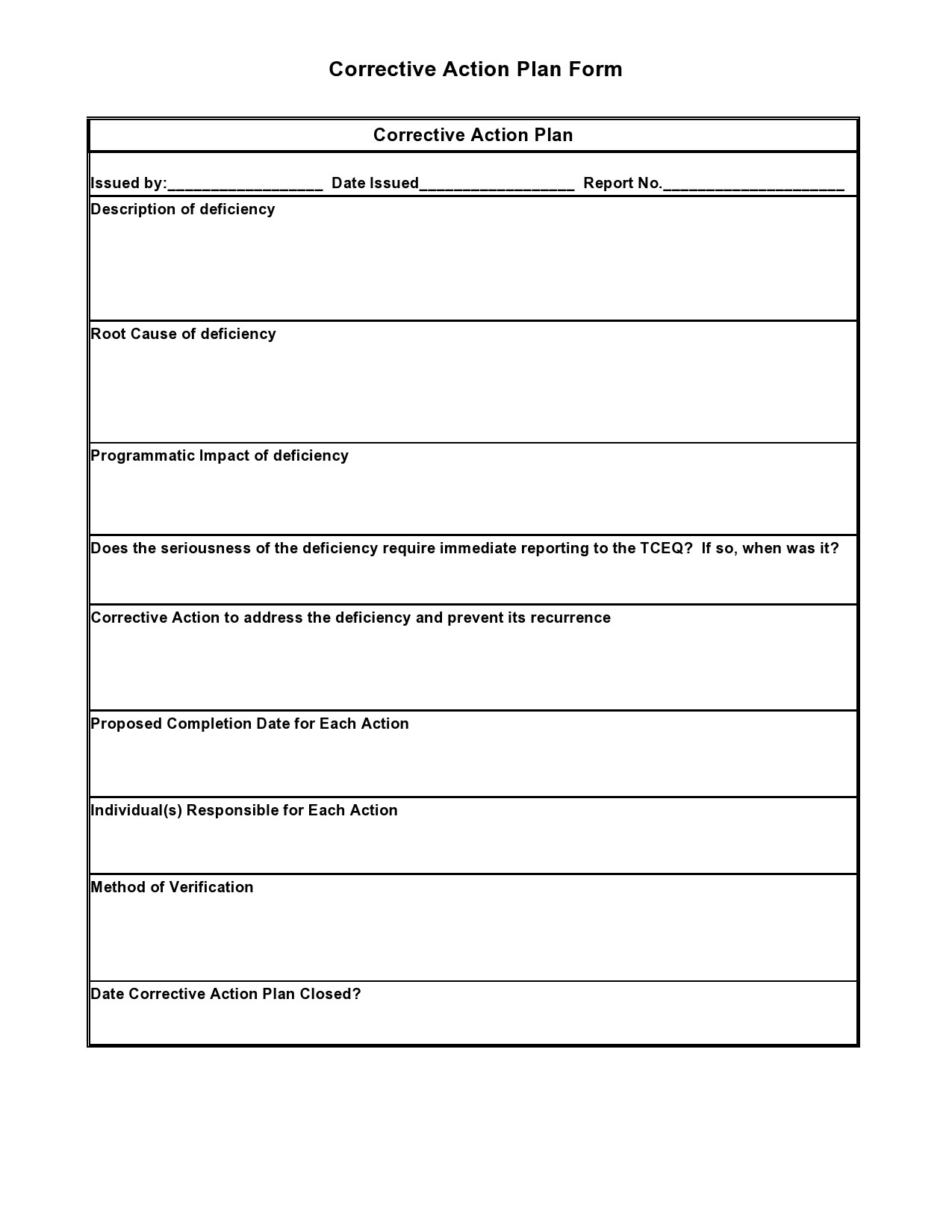
https://www.quality-assurance-solutions.com/images/xcorrective-action-form-ppt.jpg.pagespeed.ic.57vr5amThP.jpg
Under the Therapeutic Goods Act 1989 therapeutic goods may not be imported into Australia unless they are registered listed or included in the Australian Register of Therapeutic Goods Unless a specific exemption applies medical devices must be included on the Australian Register of Therapeutic Goods ARTG before they can be supplied in Australia Can health
Jan 8 2023 nbsp 0183 32 The FAQ s were related to important topics such as Regulation Importing medical devices Health professionals as sponsors Trade exhibitions Penalties Suspect devices You will be required to comply with various requirements if you intend to import into or export from Australia therapeutic goods and or biologicals There are strict requirements that apply
More picture related to Corrective Action Plan Steps
Printable Corrective Action Plan Template Fillable Form 2023
http://fillableforms.net/wp-content/uploads/2023/01/44-best-corrective-action-plan-templates-word-excel.jpg
Corrective Action Process
https://www.quality-assurance-solutions.com/images/corrective-action-forms-ppt.jpg
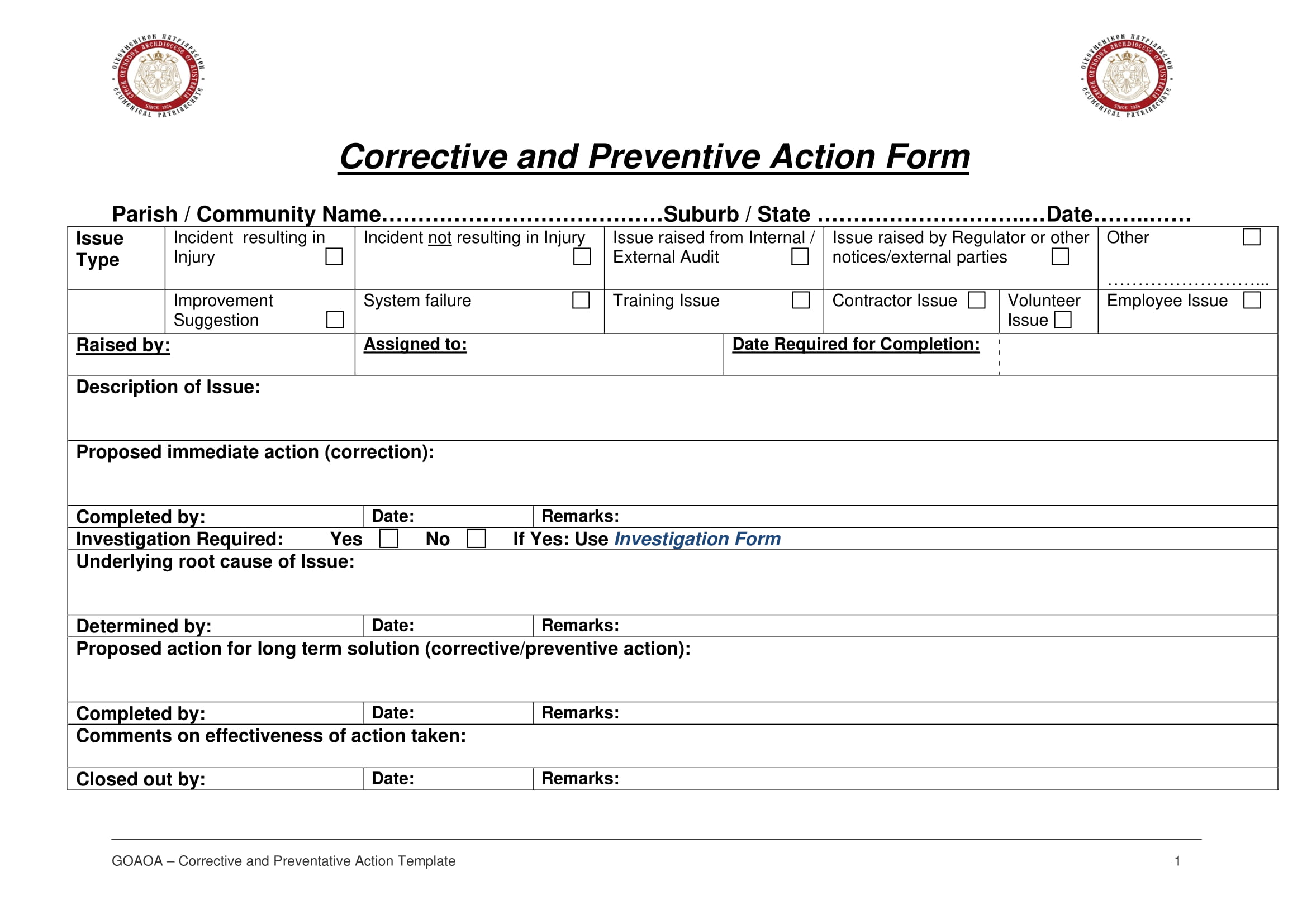
Corrective Action Report Template Atlanticcityaquarium
https://images.examples.com/wp-content/uploads/2018/08/Corrective-and-Preventive-Action-Report-Form-Example-1.jpg
Oct 31 2020 nbsp 0183 32 Australia has strict laws and regulations that govern the import and sale of therapeutic goods Your products need to be approved by the Therapeutical Goods Clinical trials involving unapproved therapeutic goods may be conducted in Australia under 2 schemes Clinical Trial Notification or Clinical Trial Approval
[desc-10] [desc-11]
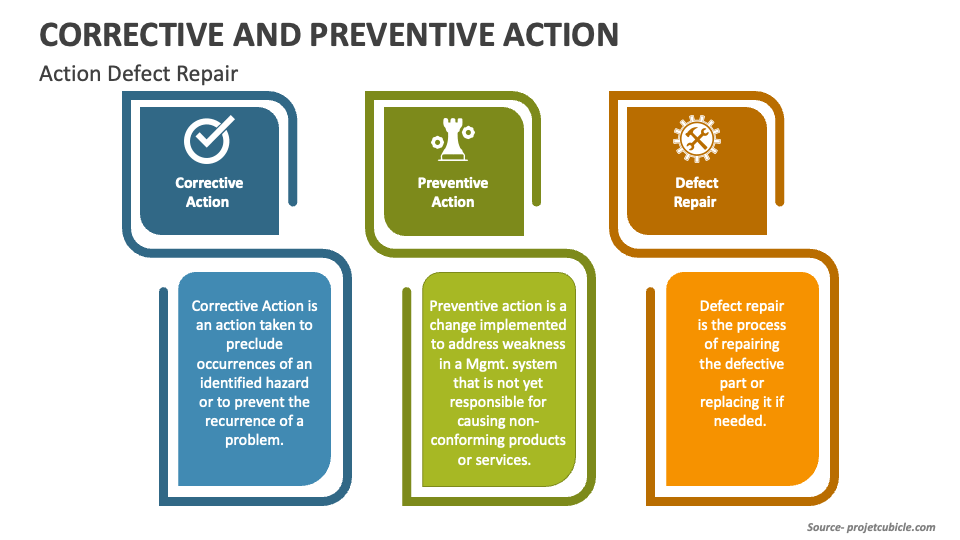
Telegraph
https://www.collidu.com/media/catalog/product/img/5/b/5bf1f3d4848fa4e17530242921041a8b7c6be3d3868bfc037aceb4b94d537c69/corrective-and-preventive-action-slide1.png
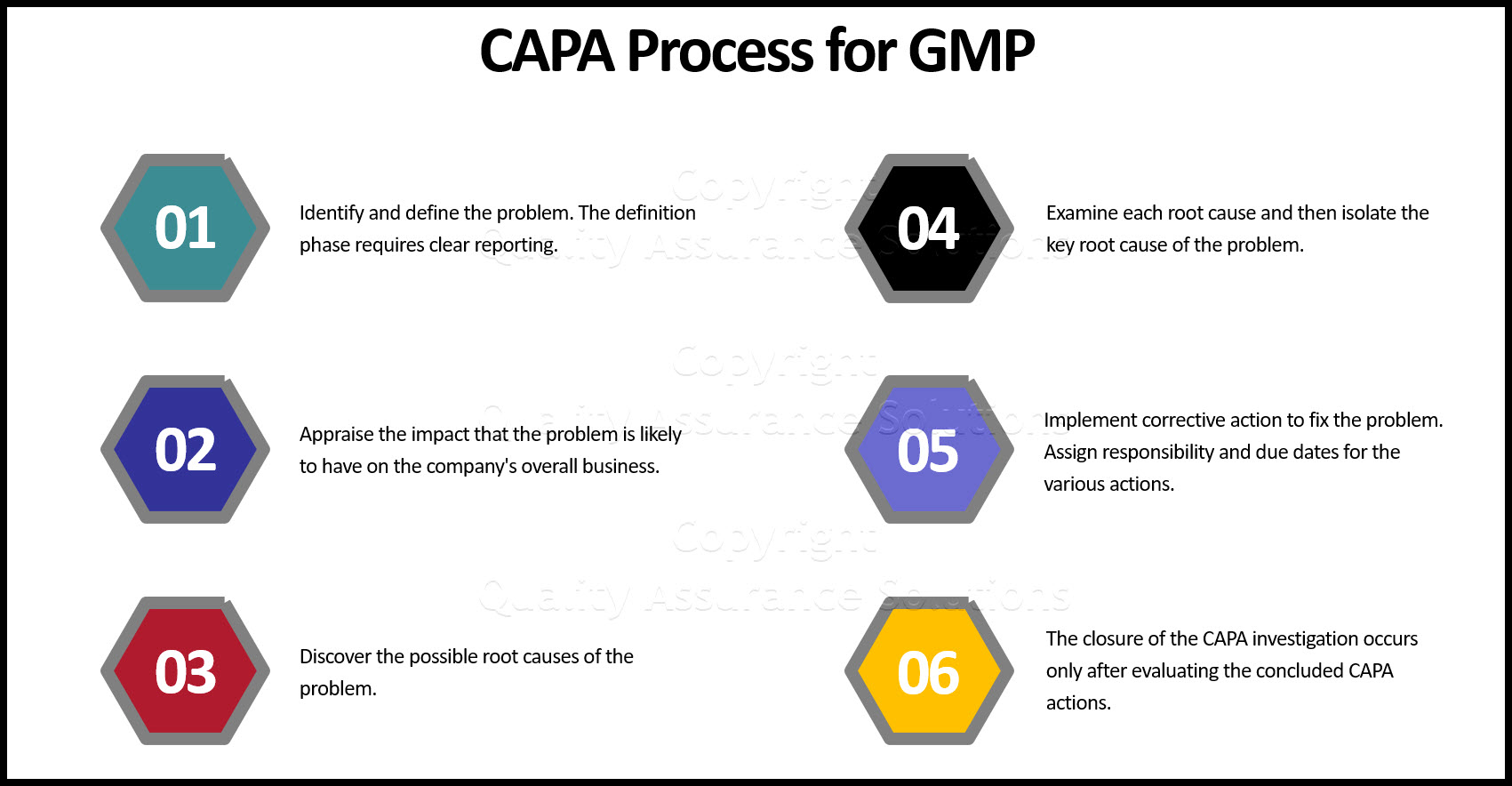
Corrective Action Process
https://www.quality-assurance-solutions.com/images/Preventive-Corrective-Action-ppt.jpg
Corrective Action Plan Steps - [desc-13]