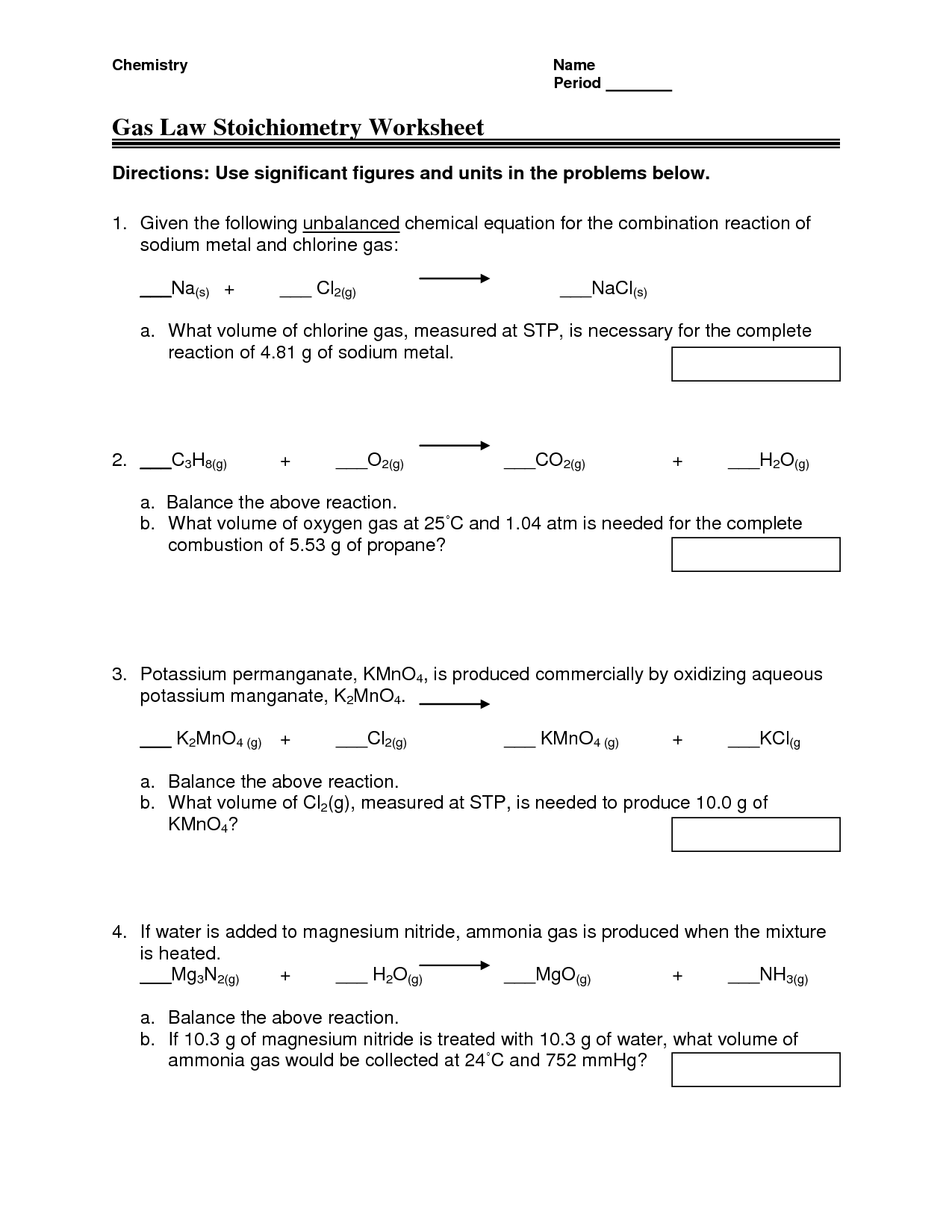
Combined Gas Law Worksheet With Answers Chemistry The Combined Gas Law Solve the following problems As always include enough work and show the units to ensure full credit 1 The pressure of a gas changes from 120 kPa to 50 kPa The volume changes from 45 L to 40 L If the initial temperature is 81oC what is the final temperature in oC 2
THE COMBINED GAS LAW Use the combined gas law to solve the following problems 1 If I initially have a gas at a pressure of 12 atm a volume of 23 liters and a temperature of 200 K and then I raise the pressure to 14 atm and increase the temperature to 300 K what is the new volume of the gas 2 A gas takes up a volume of 17 liters has a Combined Gas Law Worksheet 1 If I initially have 4 0 L of a gas at a pressure of 1 1 atm what will the volume be if I increase the pressure to 3 4 atm 2 A toy balloon has an internal pressure of 1 05 atm and a volume of 5 0 L
Combined Gas Law Worksheet With Answers
Combined Gas Law Worksheet With Answers
https://www.worksheeto.com/postpic/2015/02/gas-law-stoichiometry-worksheet_223892.png

Combined Gas Law Overview Calculations Expii
https://d20khd7ddkh5ls.cloudfront.net/combined_gas_law_formula_sheet.jpeg

20 Ideal Gas Law Worksheet Answers Worksheets Decoomo
https://i2.wp.com/www.worksheeto.com/postpic/2014/12/ideal-gas-law-worksheet-answers_223560.png
Combined Gas Law Worksheet Solutions 1 If I initially have 4 0 L of a gas at a pressure of 1 1 atm what will the volume be if I increase the pressure to 3 4 atm Mixed Gas Laws Worksheet 1 How many moles of gas occupy 98 L at a pressure of 2 8 atmospheres and a temperature of 292 K 2 If 5 0 moles of O2 and 3 0 moles of N2 are placed in a 30 0 L tank at a temperature of 250 C what will the
The following practice problems are to master to topics on the ideal gas laws Boyle s law Charles s law and Avogadro s Law as well as the combined gas law equation There are examples to work on the Dalton law of partial pressures the Graham s law of A gas balloon has a volume of 106 0 liters when the temperature is 45 0 176 C and the pressure is 740 0 mm of mercury What will its volume be at 20 0 176 C and 780 0 mm of mercury pressure
More picture related to Combined Gas Law Worksheet With Answers

Ideal Gas Law Practice Worksheet
https://s3.studylib.net/store/data/008423281_1-c32115dedb373c7f8ffe4fb72e5478df.png
Worksheet Mixed Gas Law Worksheet
https://s3.studylib.net/store/data/009221976_1-66e6457ca396ab87cae6eba038b452e2.png
Gas Laws Worksheet 1 Answer Key Db excel
https://db-excel.com/wp-content/uploads/2019/09/worksheet-gas-laws-ii-answers-9-728x942.png
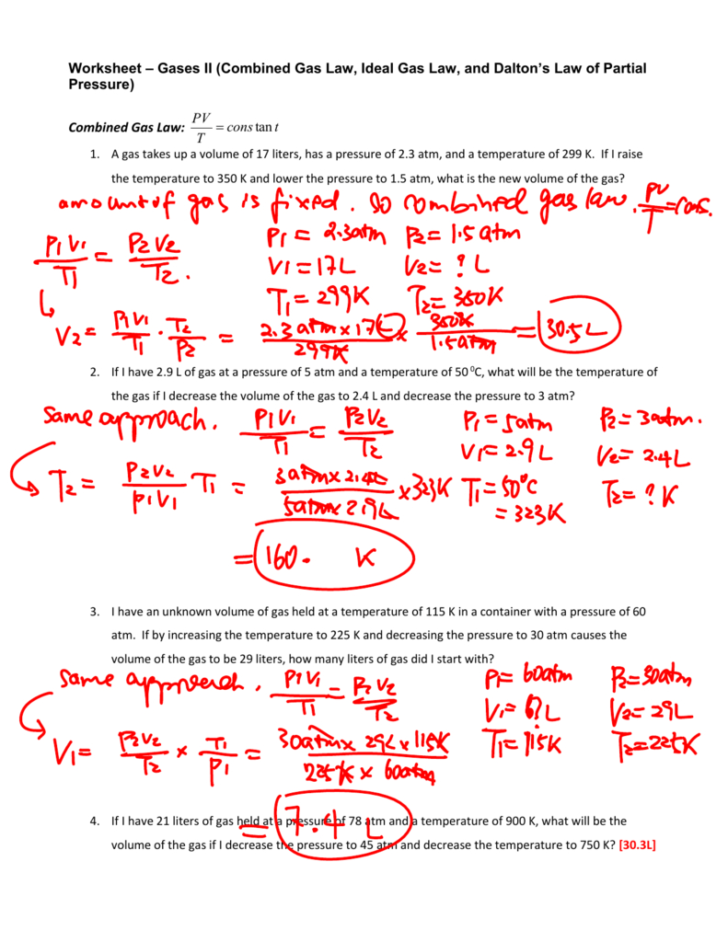
If I have 21 litres of gas held at a pressure of 78 atm and a temperatre of 900K what will be the volume of the gas if I decrease the pressure to 45 atm and decrease the temperature to 750K What will be the volume of the same gas at 745 0 torr and 30 0 oc 6 A gas occupies a volume of 34 2 mL at a temperature of 15 0 oc and a pressure of 800 0 torr
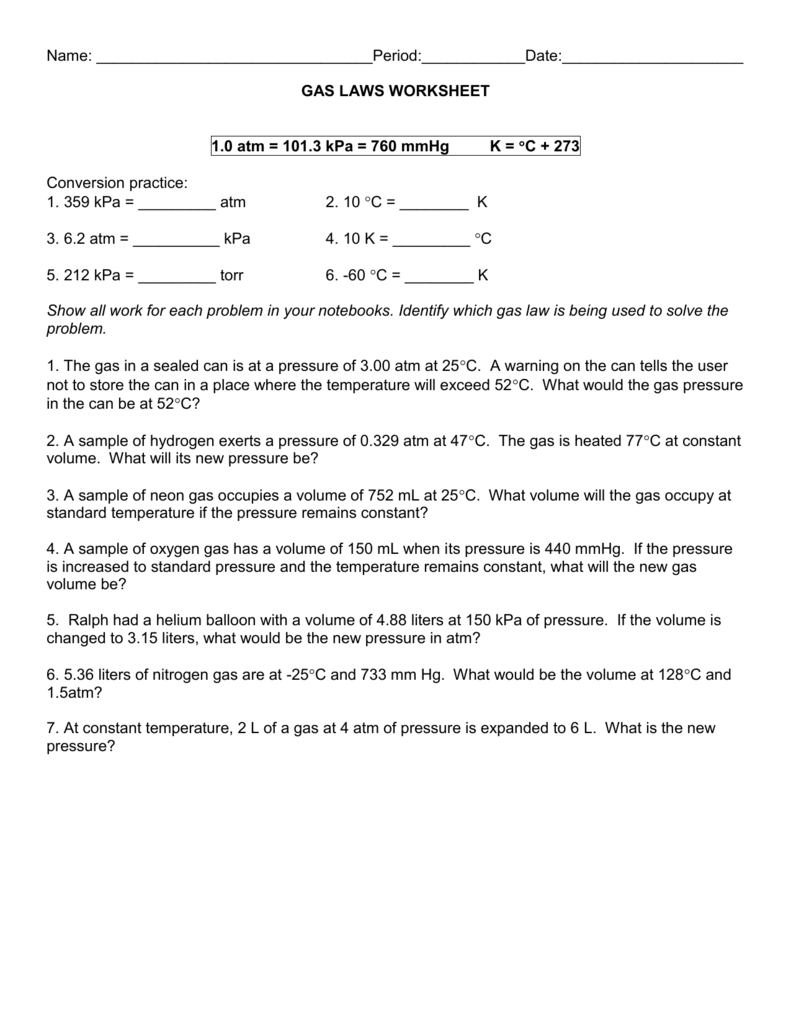
Identify the appropriate gas law to use under the given conditions and use it to solve for an unknown quantity Includes 10 problems Use the combined gas law to solve the following problems 1 If I initially have a gas at a pressure of 12 atm a volume of 23 liters and a temperature of 200 K and then I raise the pressure to 14 atm and increase the temperature to 300 K what is the new volume of the gas
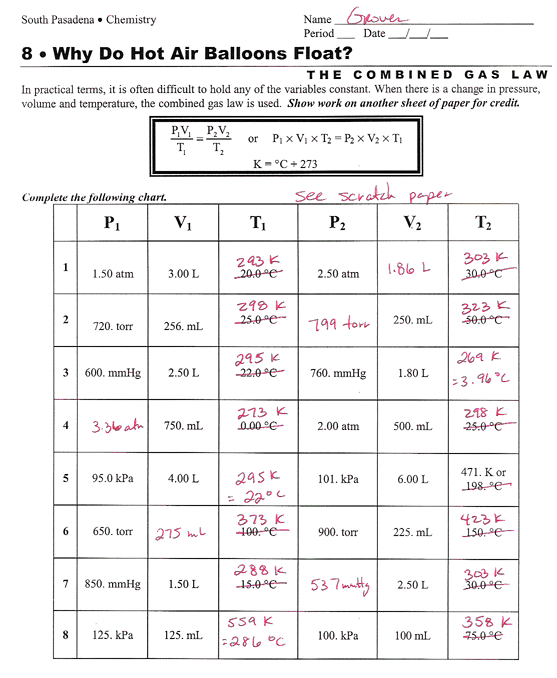
Gen Chem Page
http://www.chemmybear.com/groves/images/gcunit08_comb1.gif
Determining Gas Properties Through Gas Law Calculations PDF Gases
https://imgv2-2-f.scribdassets.com/img/document/247039702/original/19a05f6b4c/1705703005?v=1
Combined Gas Law Worksheet With Answers - The following practice problems are to master to topics on the ideal gas laws Boyle s law Charles s law and Avogadro s Law as well as the combined gas law equation There are examples to work on the Dalton law of partial pressures the Graham s law of
