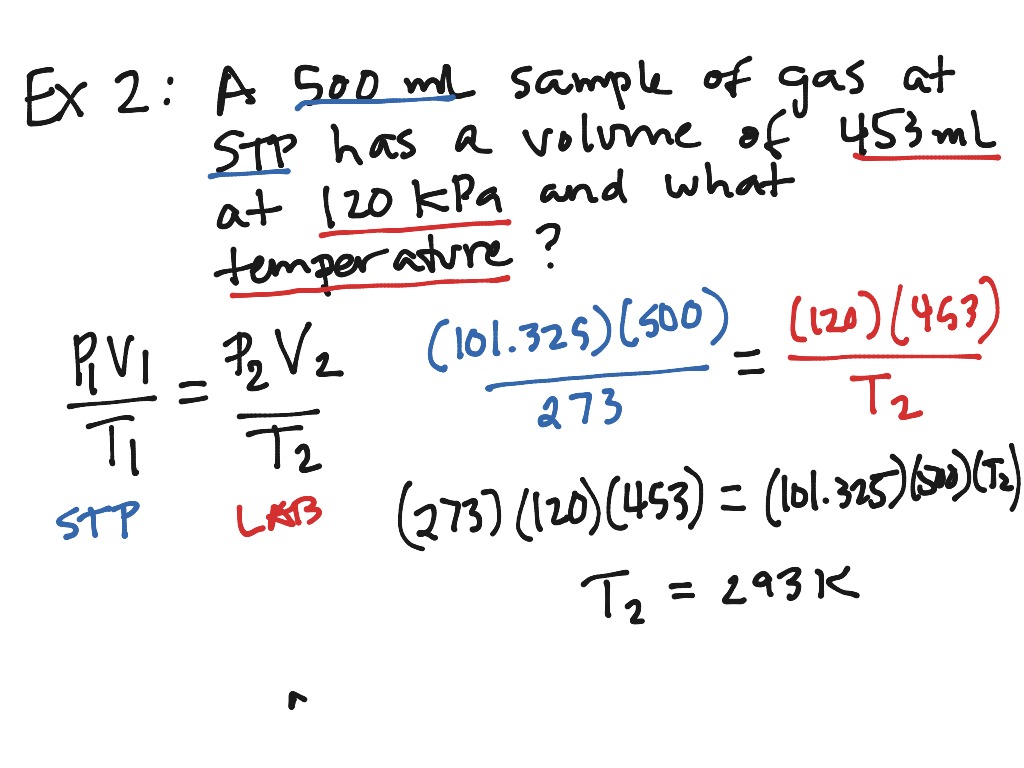
Combined Gas Law Example Problem 1 A gas has a volume of 800 0 mL at 23 0 176 C and 300 0 torr What would the volume of the gas be at 227 0 176 C and 600 0 torr of pressure 1 Set up all the problem values in a solution matrix T 1 250 T 2 500 2 The combined gas law is rearranged to isolate V 2 3 Values are inserted into the proper places
What is Combined Gas Law The combined gas law is the law which combines Charles s law Gay Lussac s law and Boyle s law It s an amalgamation of the three previously discovered laws These laws relate one thermodynamic variable to another holding everything else constant Example 0 What gas law would you use if the pressure volume and moles changed while the temperature remained constant Solution You would use an unusual form of the combined gas law 1 Write the full Combined Gas Law
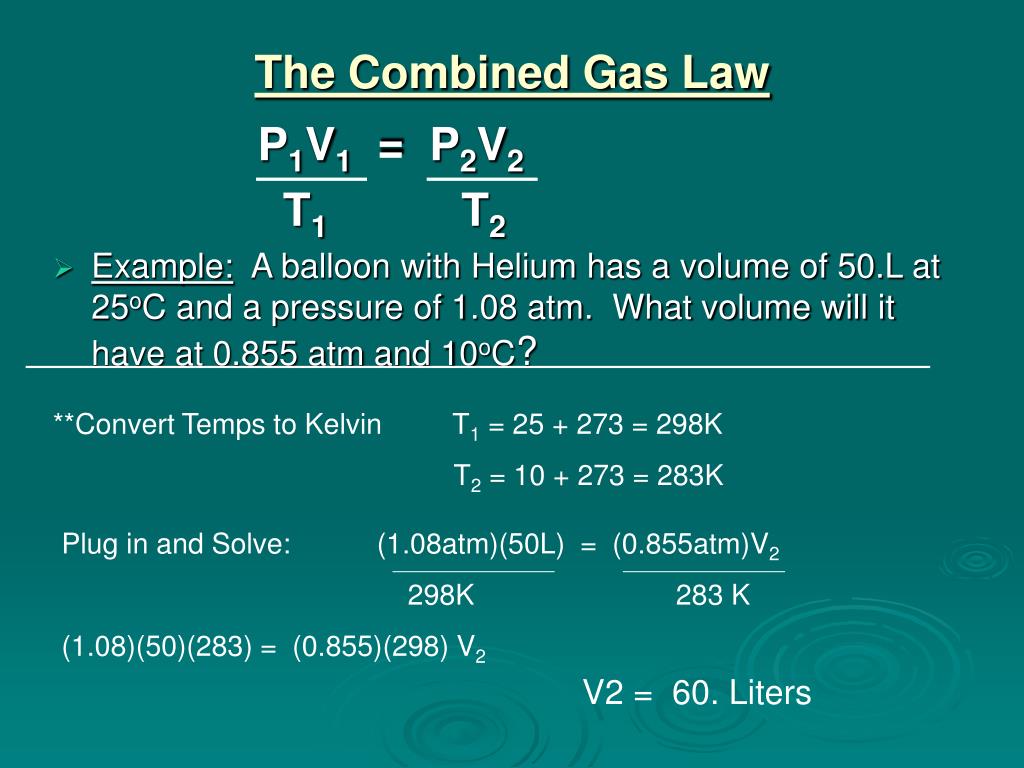
Combined Gas Law Example
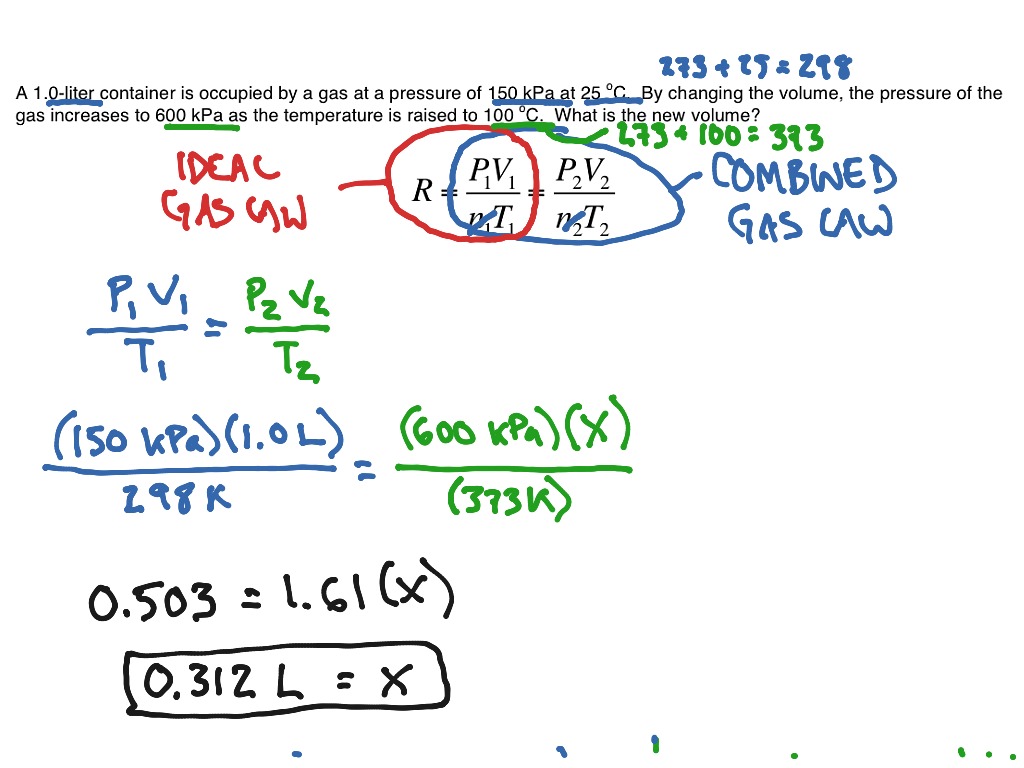
Combined Gas Law Example
https://showme0-9071.kxcdn.com/files/1000379512/pictures/thumbs/2610196/last_thumb1488319550.jpg

Solving Combined Gas Law Problems Charles Law Boyle s Law Lussac s
http://i.ytimg.com/vi/CgrCFRsWMtI/maxresdefault.jpg

Combined Gas Law Definition Formula Examples Boyle s Law Ideal
https://i.pinimg.com/originals/ff/c7/a6/ffc7a6f85c4d04780f88fcbe327508c8.png
Mar 21 2025 nbsp 0183 32 The combined gas law expresses the relationship between the pressure volume and absolute temperature of a fixed amount of gas For a combined gas law problem only the amount of gas is held constant The combined gas law is one more gas law equation that can be used in the solution of gas law problems Here are three examples with accompanying solutions Additional examples are provided in the Check Your Understanding section
Graham s Law of Effusion The Combined Gas Law The combined gas law relates pressure temperature and volume when everything else is held constant mainly the moles of gas n The most common form of the equation for the combined gas law is as follows P is the pressure of the gas T is the temperature of the gas V is the volume of the gas The law that combines Charles law Gay Lussac s law Avogadro s law and Boyle s law is known as the combined gas law These laws connect one thermodynamic variable to another while keeping all other variables constant
More picture related to Combined Gas Law Example
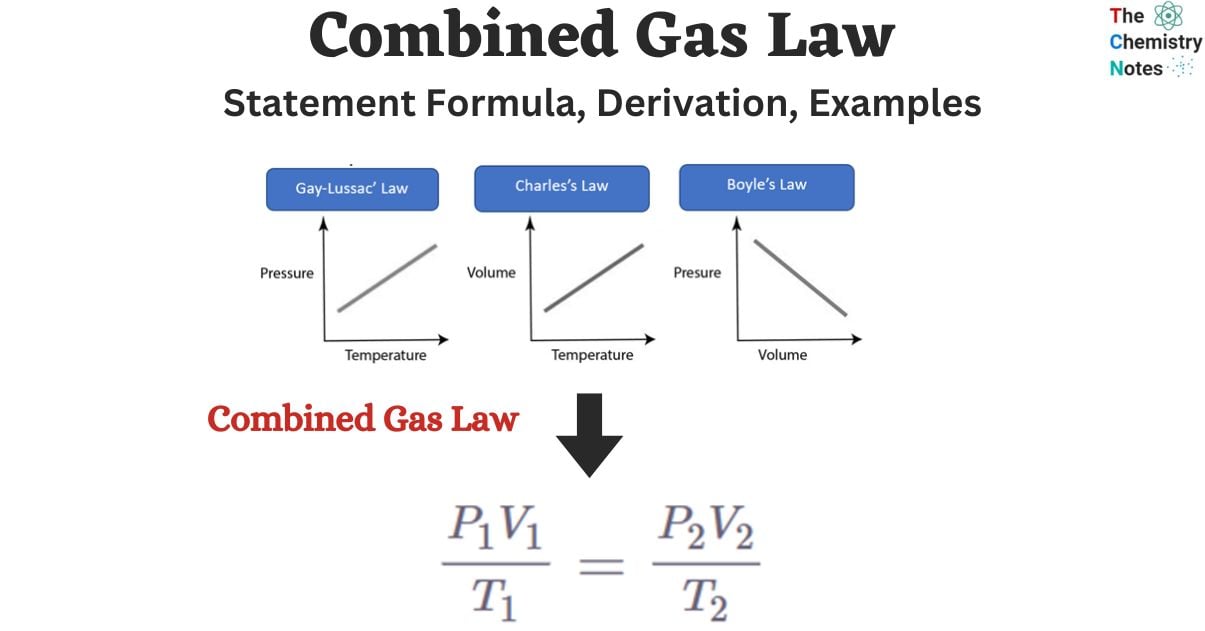
Combined Gas Law Formula Derivation Examples
https://scienceinfo.com/wp-content/uploads/2023/08/Combined-Gas-Law.jpg
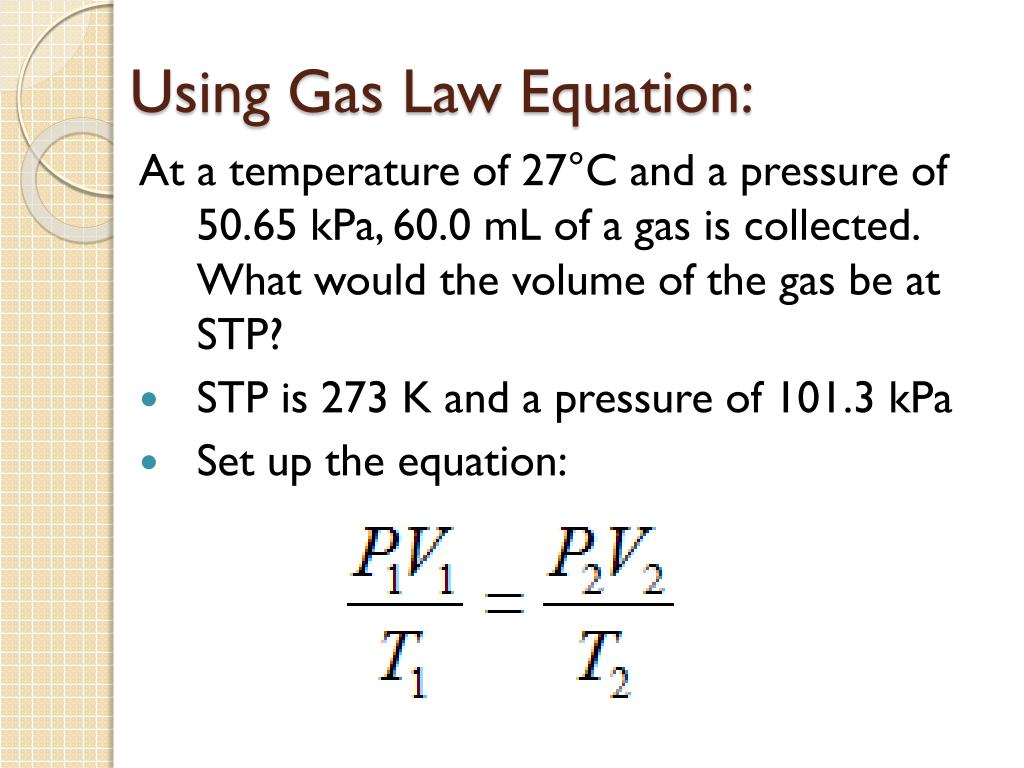
The Combined Gas Law Examples
https://image1.slideserve.com/3252378/using-gas-law-equation-l.jpg
Ideal Gas Law Equation Example
https://asiasupergrid.com/images/ideal-gas-law-equation-example.jpg
Combined Gas Law The Combined Gas Law combines Charles Law Boyle s Law and Gay Lussac s Law The Combined Gas Law states that a gas pressure 215 volume temperature constant Example A gas at 110kPa at 30 0 176 C fills a flexible container with an initial volume of Example 1 2 00 L of a gas is collected at 25 0 176 C and 745 0 mmHg What is the volume at STP Example 2 The pressure of 8 40 L of nitrogen gas in a flexible container is decreased to one half its original pressure and its absolute temperature is increased to double the original temperature What is the new volume
[desc-10] [desc-11]
When To Use Ideal Gas Law Guides Online
https://image2.slideserve.com/5321509/the-combined-gas-law-l.jpg

Combined Gas Law Definition Formula Example Video Lesson
http://study.com/cimages/videopreview/combined-gas-law-definition-formula-example_01003002_165029.jpg
Combined Gas Law Example - [desc-14]