Classifying Matter Mixtures And Pure Substances Worksheet Answers Elements Compounds and Mixtures SNC2D 1 Classify each of the following as elements E compounds C or Mixtures M Write the letter X if it is none of these
The document is a worksheet classifying different types of matter as homogeneous or heterogeneous pure substances or mixtures and checking them in multiple categories of heterogeneous homogeneous pure substance solution element compound or mixture 7 Classify the following materials as element compound pure substance homogeneous mixture or heterogeneous mixture there may be more than one answer for each j A clear liquid that does not separate into simpler substances with gentle heating it only evaporates When an electric current is used on it two gases are produced
Classifying Matter Mixtures And Pure Substances Worksheet Answers

Classifying Matter Mixtures And Pure Substances Worksheet Answers
https://i.pinimg.com/736x/03/c0/59/03c059e2bd0fa9d1ae240921786099c0.jpg

Elements Compounds And Mixtures Classification Of Matter With Images
https://i.pinimg.com/originals/a5/7f/3b/a57f3b3341c7c0a0b22aef9ad48404c3.png

Pure Substances And Mixtures Worksheets
http://www.unmisravle.com/wp-content/uploads/2018/04/mixture_problems_worksheet_1.png
Classify each of the materials below In the center column state whether the material is a pure substance or a mixture If the material is a pure substance further classify it as either an element or compound in the right column Similarly if the material is a mixture further classify it as homogeneous or heterogeneous in the right column Classify each of the following as one of an Atom A a Molecule M or an Ion I e Ge f g Ca2 h NH3 4 Describe the difference between a homogeneous and heterogeneous mixture Give an example of each 5 Assume you have 10g of pure gold Should you refer to the gold as an atom or an element Why
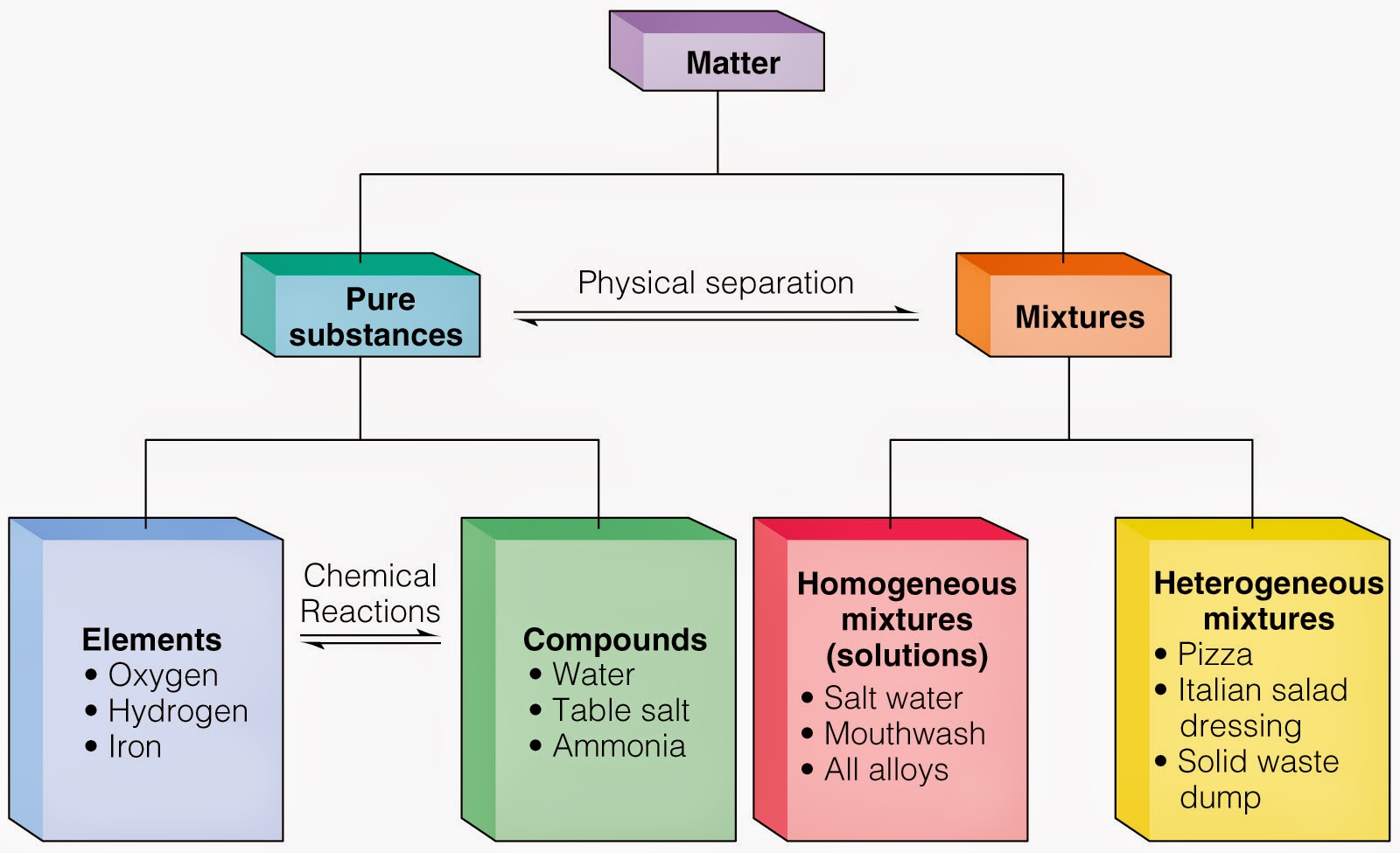
To help you answer the question let us go through the following questions 1 Write down what you see in the picture 2 Do you think it is a mixture of substances or a pure substance Why do you think so 3 Are the atoms all of the same kind 4 What class of substances is made up of only one kind of atom 5 Is the substance an element Why 6 Describe the difference between a mixture and a pure substance Pure substances are further broken down into elements and compounds while a mixture is physically combined structures that can be separated into the originally A Gold Au Substance B Vegetable soup Mixture C Saltwater Mixture D Table salt sodium chloride Mixture
More picture related to Classifying Matter Mixtures And Pure Substances Worksheet Answers
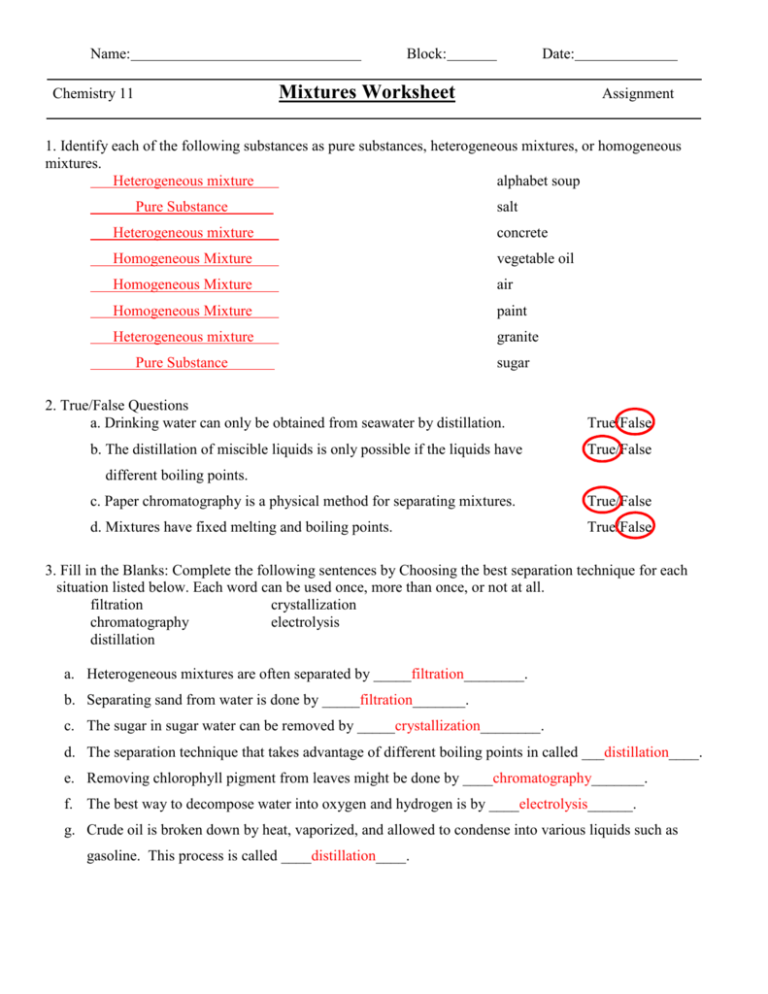
Mixtures Worksheet
https://s3.studylib.net/store/data/008171375_1-9ef18a48487e61e4fe24bb676d0fe48d-768x994.png

Substances Vs Mixtures Worksheet
https://s3.studylib.net/store/data/007131830_1-d9180483c638f78a860ddabeb86cc289-768x994.png
6th Grade Science 1st Six Weeks week 5 Pure Substances And Mixtures
https://4.bp.blogspot.com/-BAvI5pwsLDg/VQiE6fXSGaI/AAAAAAAAAgE/6FHMvCz0vgY/s1600/elements%2C%2Bcompound%2C%2Bmix2.jpg
This document contains a worksheet classifying different types of matter as either homogeneous or heterogeneous pure substances or mixtures and identifying them according to several categories including element compound solution and mixture The document classifies different types of matter as either homogeneous or heterogeneous pure substances or mixtures and then further classifies substances into categories like homogeneous matter heterogeneous matter pure substances compounds elements mixtures and solutions
[desc-10] [desc-11]

Mixtures Worksheet For Grade 7
https://s2.studylib.net/store/data/025618819_1-e98ce04485bcb53d99b5e8eaaa266b34-768x994.png

Pure Substance Vs Mixture Worksheet Ivuyteq
https://s3.studylib.net/store/data/006934129_1-6071de0f4bc6d20fb5c7636357661dd2.png
Classifying Matter Mixtures And Pure Substances Worksheet Answers - Classify each of the following as one of an Atom A a Molecule M or an Ion I e Ge f g Ca2 h NH3 4 Describe the difference between a homogeneous and heterogeneous mixture Give an example of each 5 Assume you have 10g of pure gold Should you refer to the gold as an atom or an element Why