Chemistry Valence Electrons Lewis Dot Structures Worksheet Write the element symbol Carbon is in the 4th group so it has 4 valence electrons Starting at the right draw 4 electrons or dots counter clockwise around the element symbol Check your work Using your periodic table check that Carbon is in the 4th group You should have 4 total electrons or dots drawn in for Carbon
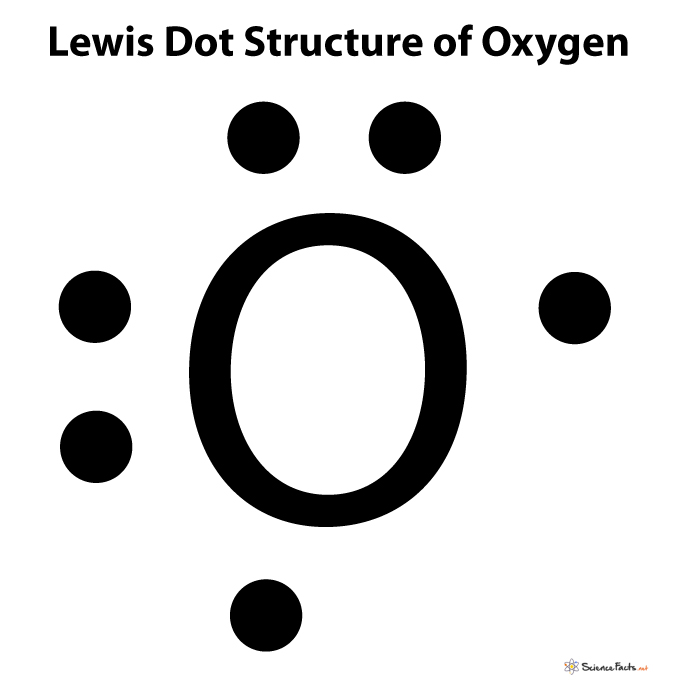
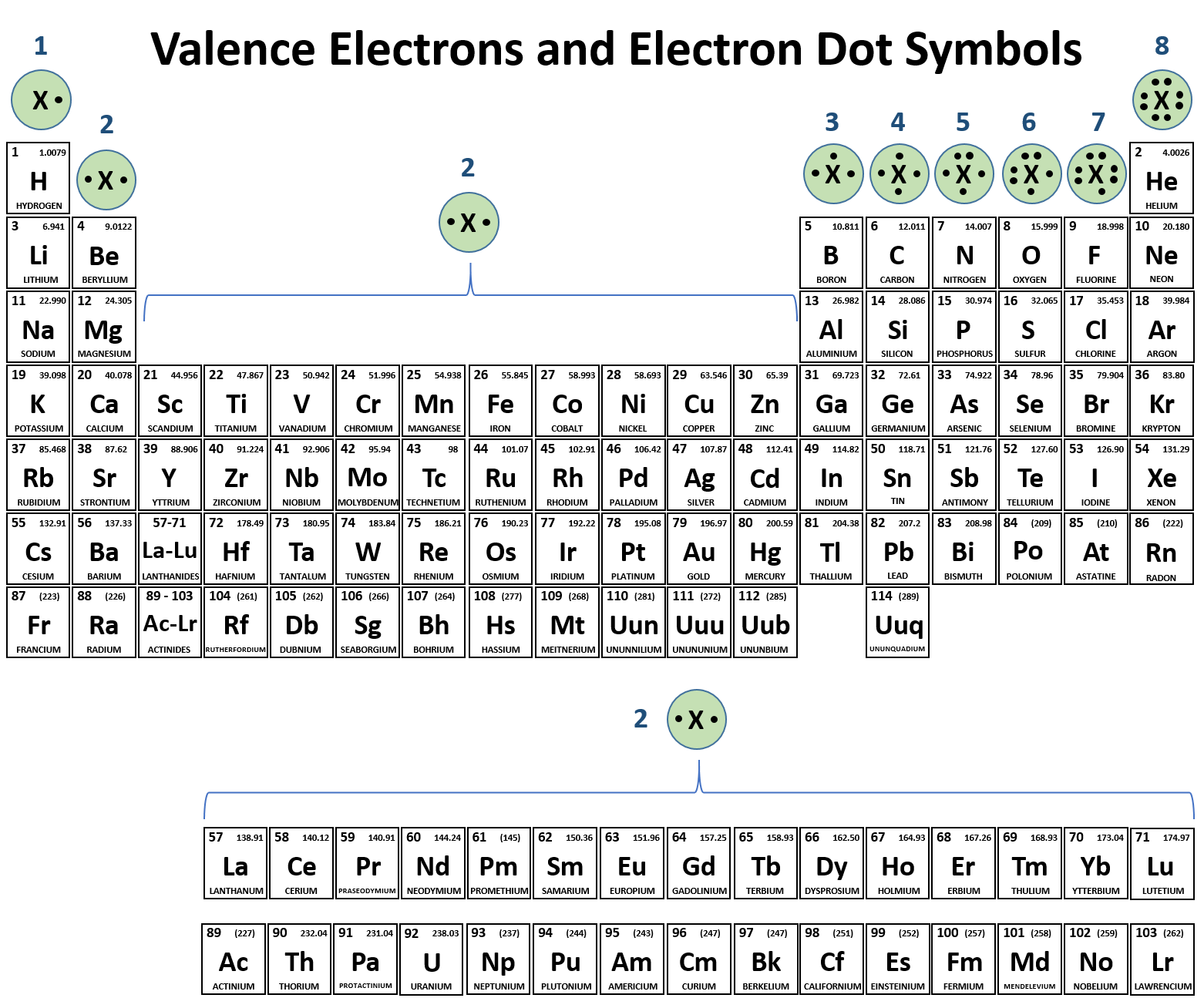
Lewis Symbols We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons Figure 7 9 shows the Lewis symbols for the elements of the third period of the periodic table Step 1 Figure out how many electrons the molecule must have based on the number of valence electrons in each atom When drawing the structure of an ion be sure to add subtract electrons to account for the charge Step 2 Connect the atoms to each other with single bonds to form a skeleton structure
Chemistry Valence Electrons Lewis Dot Structures Worksheet
Chemistry Valence Electrons Lewis Dot Structures Worksheet
https://s2.studylib.net/store/data/010212602_1-7d40b2312f1ea3c3e061ca00f159c411.png

a Write Electron Dot Structures Of Ca At No 20 And O At No 8
https://cdn.entrance360.com/media/uploads/2020/09/22/grd.png
Valence Electrons Definition Location Importance And Diagram
https://www.sciencefacts.net/wp-content/uploads/2021/11/Valence-Electrons-Lewis-Dot-Structure.jpg
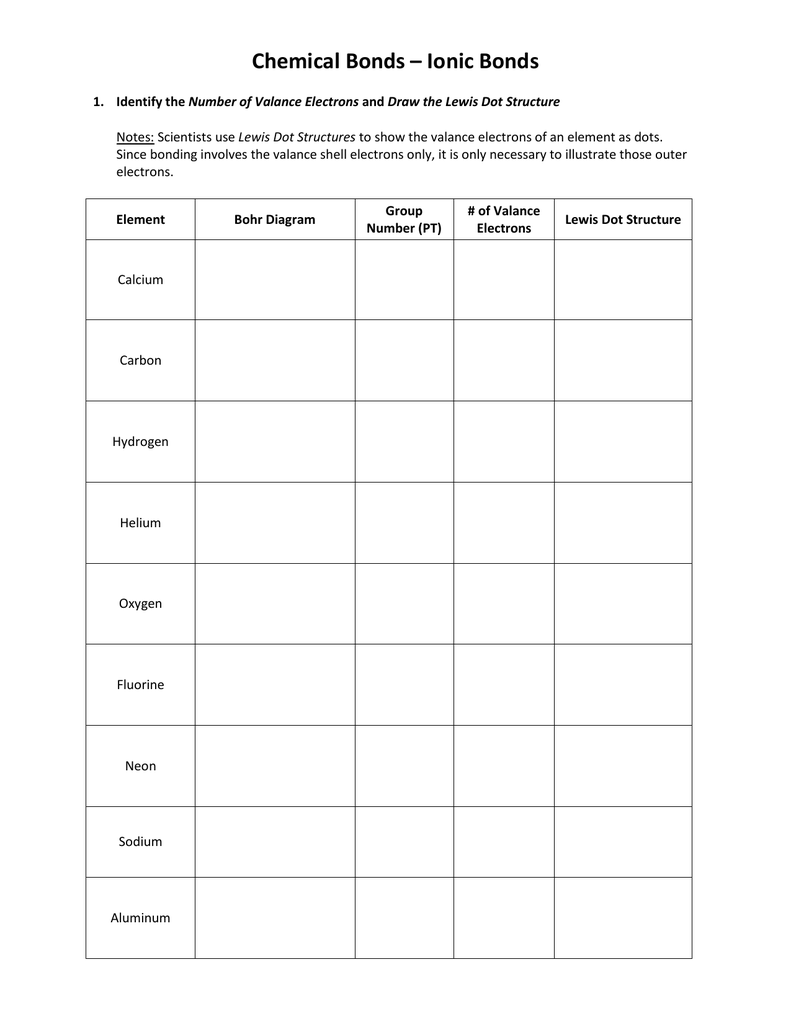
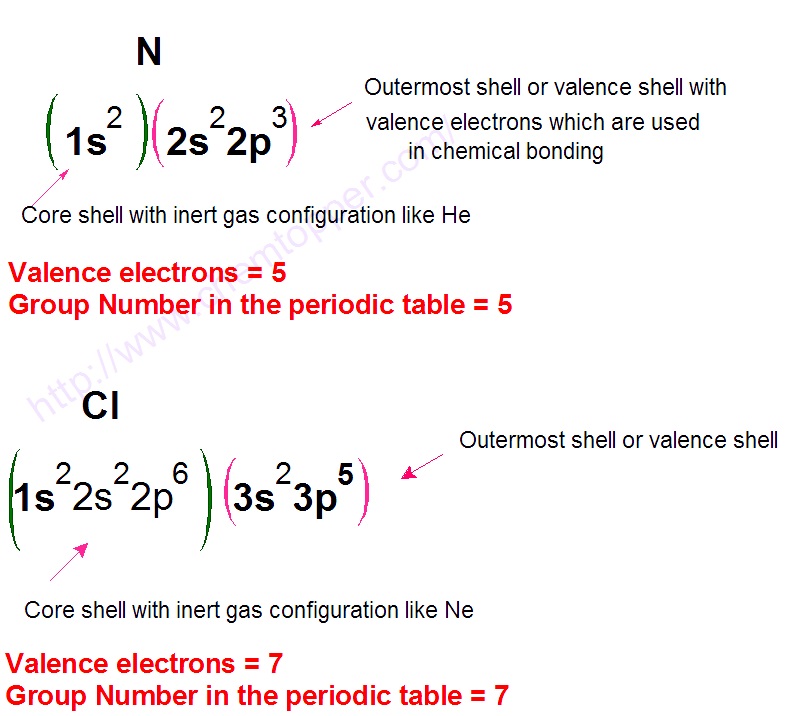
These structures also known as lewis structures or electron dot structures are drawings that visually demonstrate how electrons are shared and arranged around atoms The electrons denoted as dots are called lone pairs and belong to an individual atom Electrons denoted as lines are bonds and show the sharing of two electrons between two atoms A Lewis electron dot symbol or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element The number of dots equals the number of valence electrons in the atom These dots are arranged to the right and left and above and below the symbol
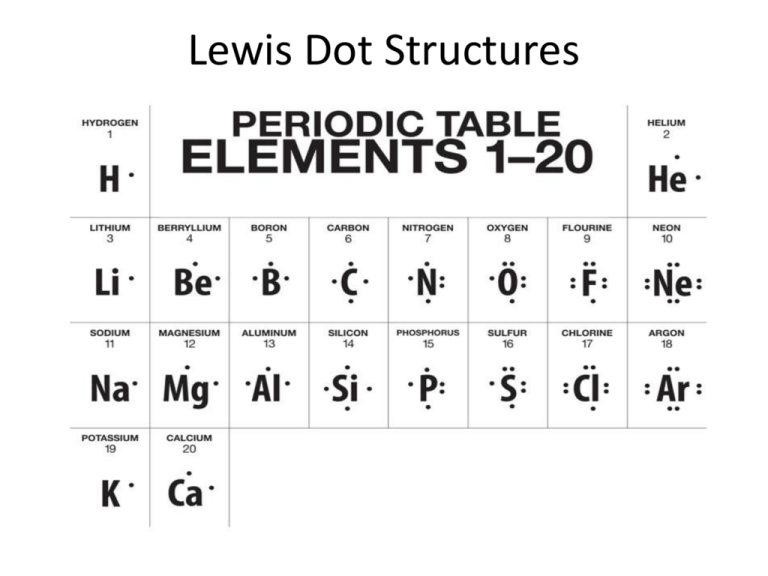
A Lewis or Electron Dot Structure is a convenient representation of the valence electrons in an atom An electron dot structure for an atom is simply the symbol for the element surrounded by a number of dots equal to the number of valence electrons Avoid a common mistake the dots represent valence electrons only so make sure you use only Work in groups on these problems You should try to answer the questions without referring to your textbook If you get stuck try asking another group for help For each of the following draw the Lewis dot structure give the electron arrangement E A and the molecular geometry M G PF5 P F 5
More picture related to Chemistry Valence Electrons Lewis Dot Structures Worksheet
Lewis Dot Structures
https://s3.studylib.net/store/data/009496192_1-dee971b8a04322a994e35d2c4883914f-768x994.png

Lewis Dot Structure Ionic Compounds
https://api.www.labxchange.org/api/v1/xblocks/lb:LabXchange:0ec2747c:html:1/storage/asset-v1:LabXchange+100+2019+type@asset+block@Box_2_ch_1_example_-10de69d6fddaa0f240bb0300e68f48f9.png
How To Draw Lewis Dot Structure Online Chemistry Tutor
http://www.chemtopper.com/myblog/wp-content/uploads/2016/02/valence-electrons.jpg
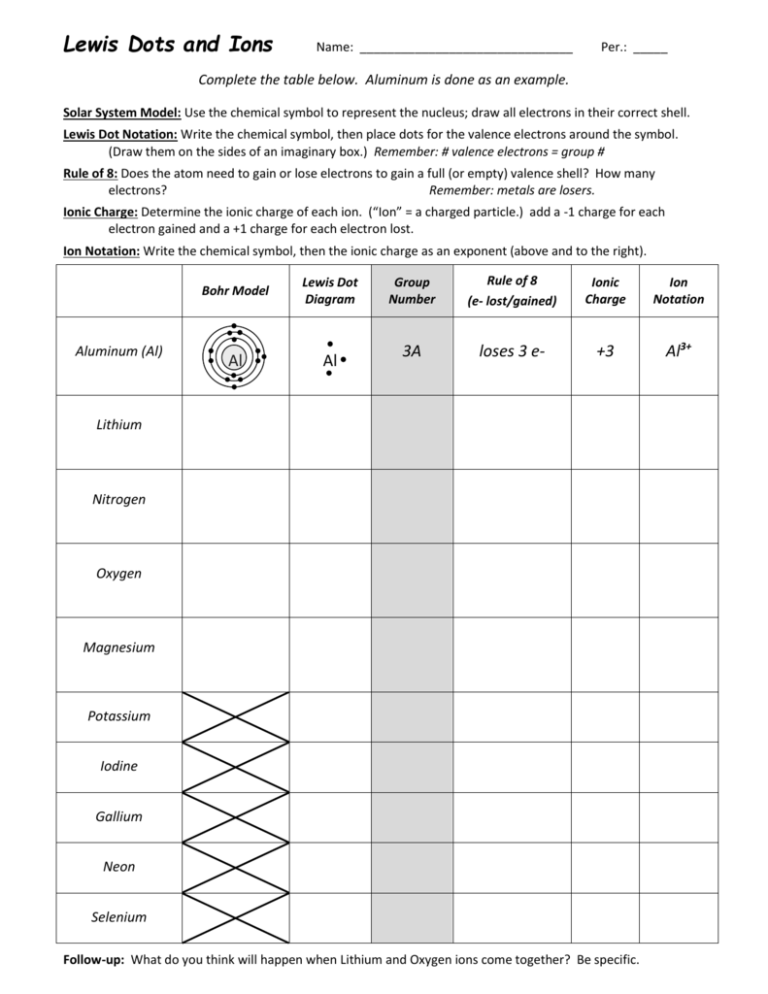
The worksheets consist of Guided notes including a key and a blank sheet for students Multiple examples of each problem type including Lewis Dot Structures Full pages of practice problems asking students to identify the number of valence electrons draw Lewis Dot Structures and identify the number of electrons that each element will gain Chemistry Valence Electrons Lewis Dot Structures Worksheet Apply your knowledge of valence electrons Lewis dot structures and the octet rule to complete the table below Boron Aluminum Carbon Silicon Lead Nitrogen Phosphorous Valence Electrons Main E Level How Many Lewis Dot Structure to achieve a full valence shell of e s gained 3
Lewis Structure Examples The Lewis electron dot structures of a few molecules are illustrated in this subsection 1 Lewis Structure of CO2 The central atom of this molecule is carbon Oxygen contains 6 valence electrons which form 2 lone pairs Since it is bonded to only one carbon atom it must form a double bond 2 Draw the Lewis dot structures for each of the following molecules a H 2 S c SO 3 b CH 2 Br 2 d HCN 3 Draw the Lewis dot structure for each of the following polyatomic ions a NH 4 c PO 4 3 b NO 3 d CO 3 2 4 For the following molecules or ions where the central atom is underlined i Draw the Electron dot structure ii
CH150 Chapter 3 Ions And Ionic Compounds Chemistry
http://www.wou.edu/chemistry/files/2017/04/valence-electrons.png
Lewis Dot Diagram Worksheet
https://s3.studylib.net/store/data/006644739_1-8452e2aa0be92fd5c883442118138132-768x994.png
Chemistry Valence Electrons Lewis Dot Structures Worksheet - A Lewis or Electron Dot Structure is a convenient representation of the valence electrons in an atom An electron dot structure for an atom is simply the symbol for the element surrounded by a number of dots equal to the number of valence electrons Avoid a common mistake the dots represent valence electrons only so make sure you use only