Chemical Reaction Vs Physical Change Oct 19 2023 nbsp 0183 32 Remember that a physical change is a change in properties such as texture shape or state while a chemical change represents the formation of a new substance after atoms are rearranged in a chemical reaction
When a chemical reaction occurs atoms are neither created nor destroyed Instead the atoms rearrange themselves to form new chemicals This is known as a chemical change The other kind of Apr 24 2017 nbsp 0183 32 Physical changes alter matter s appearance and chemical changes alter matter s composition A reaction happens when two or more molecules or groups of atoms interact The result depends on the type of molecule and how
Chemical Reaction Vs Physical Change
:max_bytes(150000):strip_icc()/physical-and-chemical-changes-examples-608338_FINAL-2-69bf88aa2f774afa8bba8df2ec203e70.png)
Chemical Reaction Vs Physical Change
https://www.thoughtco.com/thmb/cs6FHPqaVzyl1qClAJ_9cSB7cV0=/1500x0/filters:no_upscale():max_bytes(150000):strip_icc()/physical-and-chemical-changes-examples-608338_FINAL-2-69bf88aa2f774afa8bba8df2ec203e70.png

Chemical And Physical Changes Of Matter
https://sciencenotes.org/wp-content/uploads/2020/10/Chemical-and-Physical-Changes.jpg
/physical-and-chemical-changes-examples-608338_FINAL-f4e256e7fbf54f46a8c7bcefb300f5db.png)
Examples Of Physical Changes And Chemical Changes
https://www.thoughtco.com/thmb/KT6FSHraxM7wgi5hEI9ZXSpVYWY=/1500x1000/filters:fill(auto,1)/physical-and-chemical-changes-examples-608338_FINAL-f4e256e7fbf54f46a8c7bcefb300f5db.png
Chemical change both physical and chemical properties of the substance including its composition A physical change involves very little to no absorption of energy During a chemical reaction absorption and evolution of energy take Jun 4 2021 nbsp 0183 32 A physical change is a process that changes the physical form of a substance but not its chemical composition A chemical change is a process that changes the chemical composition of a substance resulting in a new compound
Learn what defines a chemical reaction indicators amp examples of a chemical reaction and physical change vs chemical change examples Chemical change is irreversible and permanent It happens when atomic bonds are broken or created during chemical reactions Physical change is a process in which a substance changes its state of matter but chemical bonds are still the
More picture related to Chemical Reaction Vs Physical Change

Distingue Cuales De Los Siguientes Son Cambios Qu micos Y Cuales De
https://es-static.z-dn.net/files/dac/b34a06dd72311c45c1bbda10ecc5c115.png

Chemical Change Diagram
https://i.pinimg.com/originals/59/1f/3c/591f3c6d0fe1943d6ac2d960f7263a09.jpg
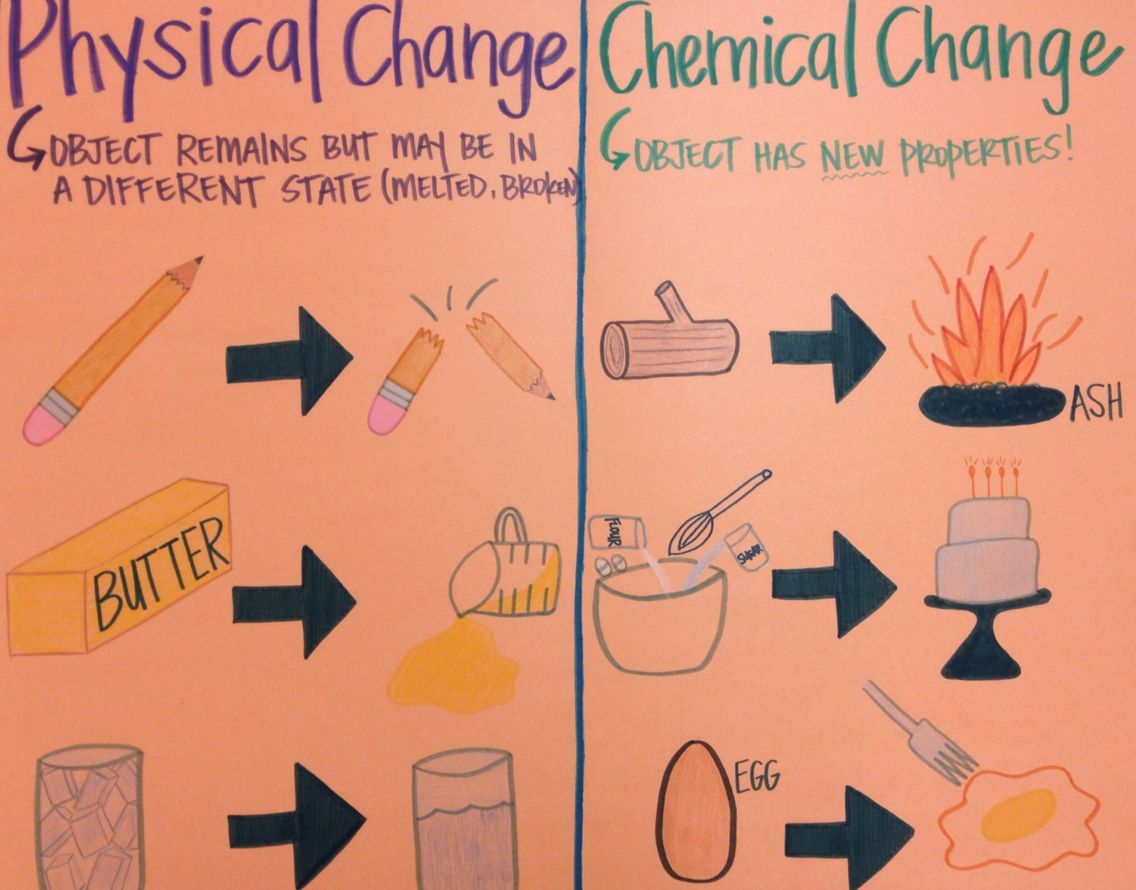
Mr Villa s 7th Gd Science Class Understanding The Difference Between
https://4.bp.blogspot.com/-3Jrxr5v8vJs/WOd6PGdYEaI/AAAAAAAAArM/gg16hgpJ7toCVAYM22rBeUxsvzH_EgqJQCLcB/s1600/Phys%2Bvs%2BChem%2Bchange2.png
May 20 2023 nbsp 0183 32 Composition distinguishes a physical reaction from a chemical reaction Matter s properties such as state of matter shape and chemical properties such as a chemical formula can endure a variety of Jul 21 2020 nbsp 0183 32 Here are the basic definitions of chemical and physical changes chemical change a process in which chemical bonds are broken or created to make a new substance physical
The difference between a physical change and a chemical change or reaction is composition In a chemical change there is a change in the composition of the substances in question in a In this lesson we will compare the characteristics of chemical and physical changes and use our knowledge to categorise changes as either physical changes or chemical reactions
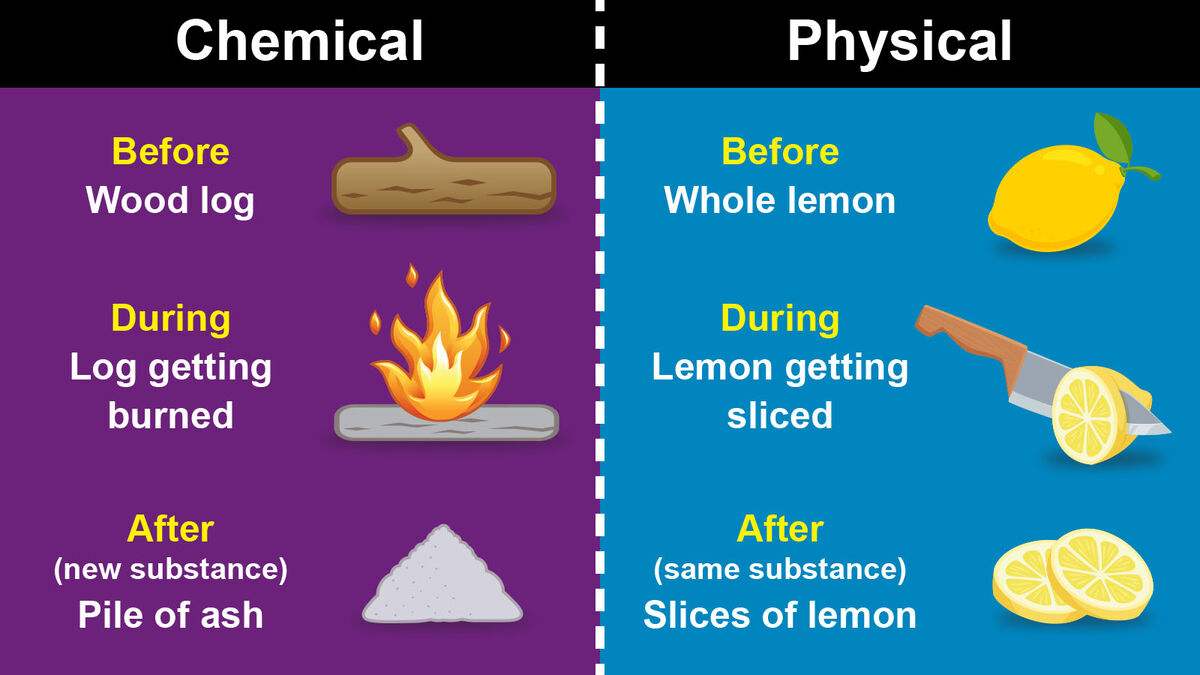
Main Difference Between A Chemical And Physical Change YourDictionary
https://assets.ltkcontent.com/images/10655/difference-chemical-physical-change_27c5571306.jpg

Difference Between Physical And Chemical Change with Examples
https://i.ytimg.com/vi/EHm6r75k1mk/maxresdefault.jpg
Chemical Reaction Vs Physical Change - Chemical change is irreversible and permanent It happens when atomic bonds are broken or created during chemical reactions Physical change is a process in which a substance changes its state of matter but chemical bonds are still the