Chemical Kinetics Worksheet Answers Given the following energy diagram for a 3 step reaction answer the questions below Which arrow indicates the activation energy for the first step of the reverse reaction Which arrow indicates the activation energy for the first step of the forward reaction
Kinetics Worksheet Answers 1 A 2 50 M solution undergoes a chemical reaction After 3 00 minutes the concentration of the solution is 2 15 M What is the rate of the reaction in M s Rate change in concentration of reactant change in time 2 5M 2 15M 180 sec 0019 M s 2 Zinc metal reacts with hydrochloric acid Oct 31 2021 nbsp 0183 32 These Chemical Kinetics Questions And Answers PDF contains the following topics Multiple Choice Questions On Chemical Kinetics With Answers PDF Rate of a Chemical Reaction Chemical Kinetics MCQ Factors Influencing Rate of a Reaction
Chemical Kinetics Worksheet Answers

Chemical Kinetics Worksheet Answers
https://www.worksheeto.com/postpic/2012/04/ap-chemistry-kinetics-worksheet_240188.png
Breathtaking Chemical Kinetics Class 12 Notes Topperlearning Predicting
https://www.studiestoday.com/sites/default/files/images11/CBSE Class 12 Chemistry Chemical Kinetics Assignment.PNG
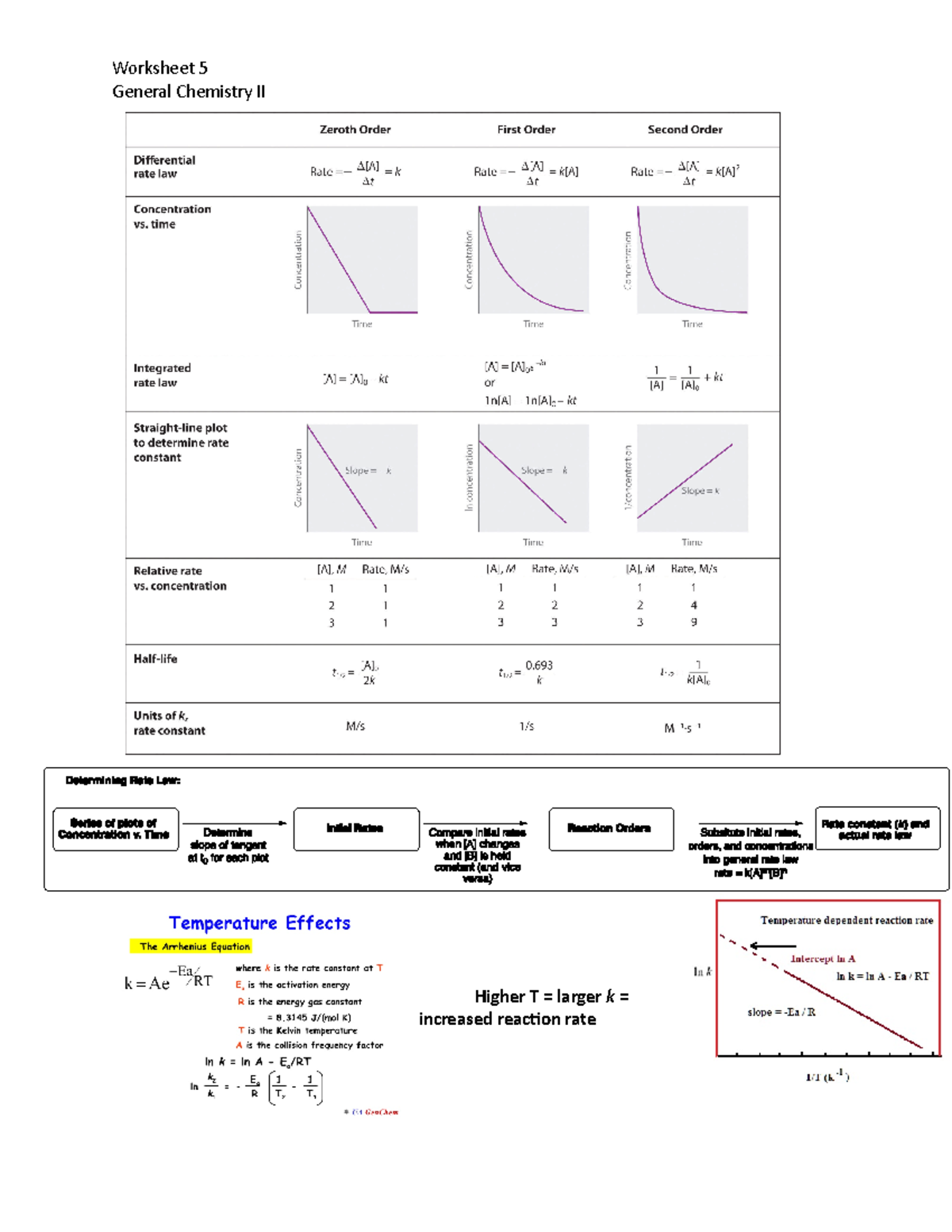
Practice Questions On Chemical Kinetics Worksheet 5 General Chemistry
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/bd41683ae6256b5f7da46a561c5ff552/thumb_1200_1553.png
CH302 Worksheet 15 on Kinetics Answer Key 1 What factors determine the rate of a reaction How does each of them affect the rate Answer Rate xAe Ea RT Shown in this equation the factors affecting rate of reaction are concentration and order of reactants and products x which increases as rate increases the activation energy Answers 1 a When we compare the results of experiments 2 and 3 we see that when B doubles the rate doubles so the reaction is first order with respect to B Experiments 2 and 3 prove that the rate must double when B doubles Knowing this we can see that when the rate doubles from experiment 1 to 2 it must be because of B and the
Download the PDF to access answers to the Chemistry Worksheet for Class 12 Chemistry Chapter 4 Chemical Kinetics Set 2 Download PDF Read Also Chemical Kinetics Theory Chemical Kinetics Questions Class 12 Chemistry Chemical Kinetics MCQs Class 12 Chemistry Chapter 4 Chemical Kinetics Important Questions With Answers The addition of a catalyst in a chemical reaction A increases the concentration of products at equilibrium B increases the fraction of reactant molecules with a given kinetic energy
More picture related to Chemical Kinetics Worksheet Answers

Class 12 Chemistry Worksheet On Chapter 4 Chemical Kinetics Set 2
https://cdn1.byjus.com/wp-content/uploads/2022/07/Chemical-Kinetics-Worksheet-Questions-Set-2.docx-2.jpg

SOLUTION Chemical Kinetics Class 12 Chemistry Cheat Sheet Studypool
https://sp-uploads.s3.amazonaws.com/uploads/services/4685183/20220818151702_62fe57eed4716_chemical_kinetics_recap.page1.jpg

SOLUTION Chemical Kinetics Explanation With Diagram And Notes Studypool
https://sp-uploads.s3.amazonaws.com/uploads/services/7029392/20230322100523_641ad2e3bc634_chemical_kineticspage0.jpg
Chemical kinetics is the study of the speed or rate of a reaction under various conditions Spontaneity is also important AND a spontaneous reaction does NOT imply a rapid reaction The changing of diamond into graphite is spontaneous but so slow that it Kinetics Practice Supplemental Worksheet KEY Determining reaction mechanism based on initial rate data 1 A reaction has the experimental rate law rate k A 2 a How will the rate change if the concentration of a is tripled If rate 1 k A 2 then rate 2 k 3A 2 32 k A 2 9 k A 2 9 rate 1 So the rate would be 9 times faster b
This document appears to be an answer key for a chemical kinetics practice test The test questions asked about activation energy values for chemical reactions and the answers given are activation energy values measured in kJ mol or J mol with the correct answer being 9 28 J mol Nov 16 2024 nbsp 0183 32 Calculate the value of k k for this reaction and express it with the correct units How would the Initial Rate change if the initial concentration of HI H I were to be non zero
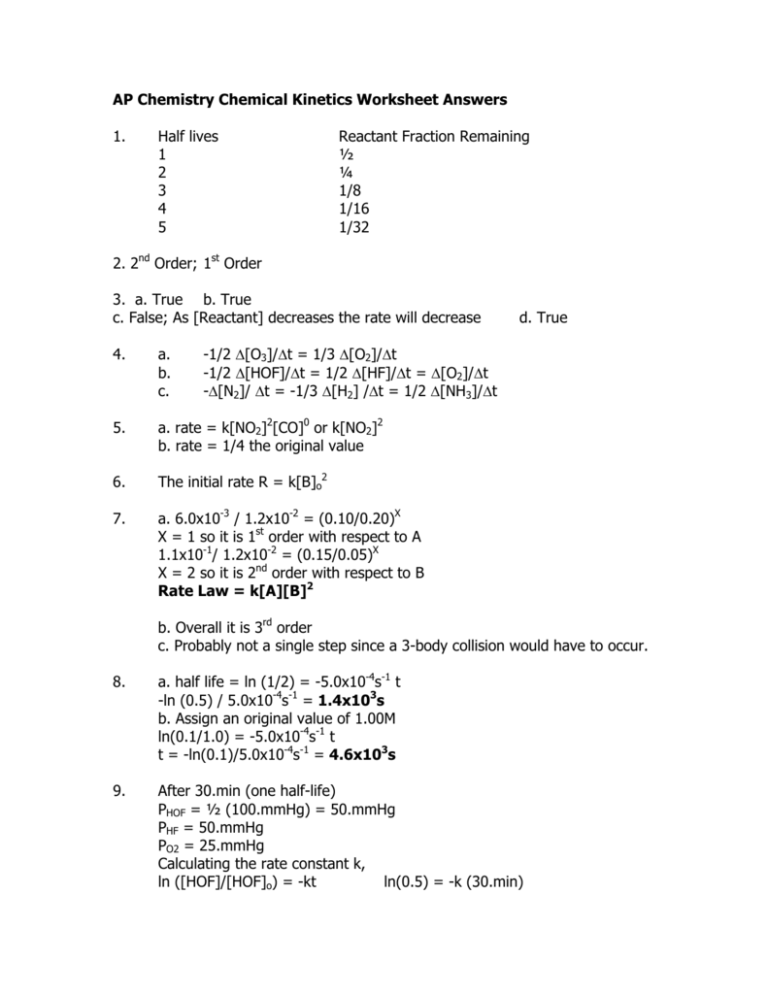
AP Chemistry Chemical Kinetics Worksheet Answers 1 Half Lives
https://s3.studylib.net/store/data/008400784_1-4c3c6901e73828908070050c13ba5b8f-768x994.png

Class 12 Chemistry Worksheet On Chapter 4 Chemical Kinetics Set 1
https://cdn1.byjus.com/wp-content/uploads/2022/07/Chemical-Kinetics-Worksheet-Questions-Set-1.docx-1-2.jpg
Chemical Kinetics Worksheet Answers - Practice Problems Chemical Kinetics Rates and Mechanisms of Chemical Reactions 1 State two quantities that must be measured to establish the rate of a chemical reaction and cite several factors that affect the rate of a chemical reaction