Attach Email To Microsoft Planner Task Question 10 Consider the set of successive ionization energies IE1IE2IE3IE4 418 8 kJ mol 3052 kJ mol 4420 kJ mol 5877 kJ mol Identify the element in period 4 that
When the difference between successive IEs of a given element is exceptionally large e g between IE1 and IE2 of K what do we learn about the element s electron configuration C In Answer to Consider the following set of successive ionization Identify the points at which there are significant jumps in the ionization energies and compare these values to known trends in
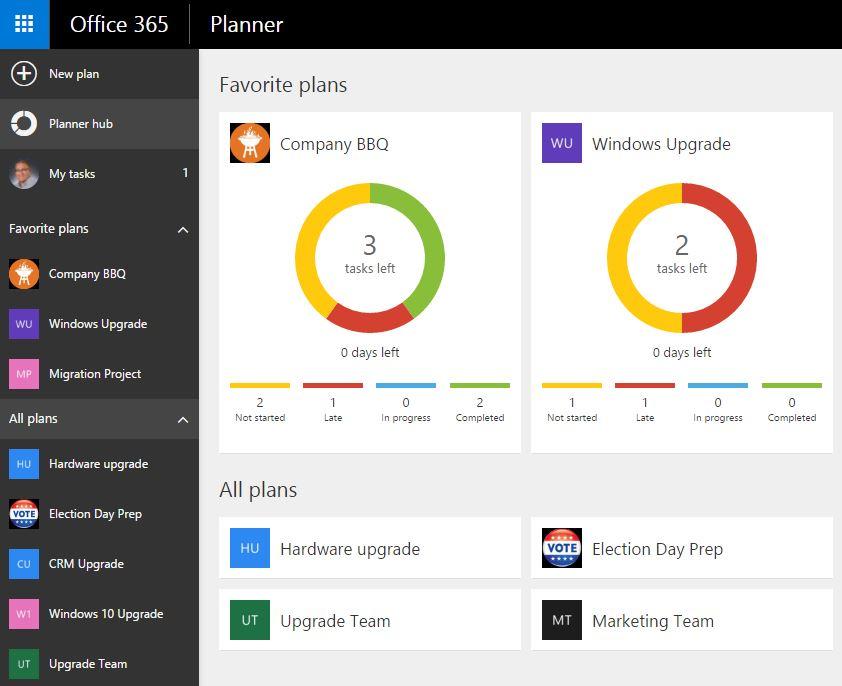
Attach Email To Microsoft Planner Task

Attach Email To Microsoft Planner Task
https://sm.pcmag.com/pcmag_uk/gallery/m/microsoft-/microsoft-planner_43j4.png
Microsoft Planner Logo LogoDix
https://logodix.com/logo/361383.jpg
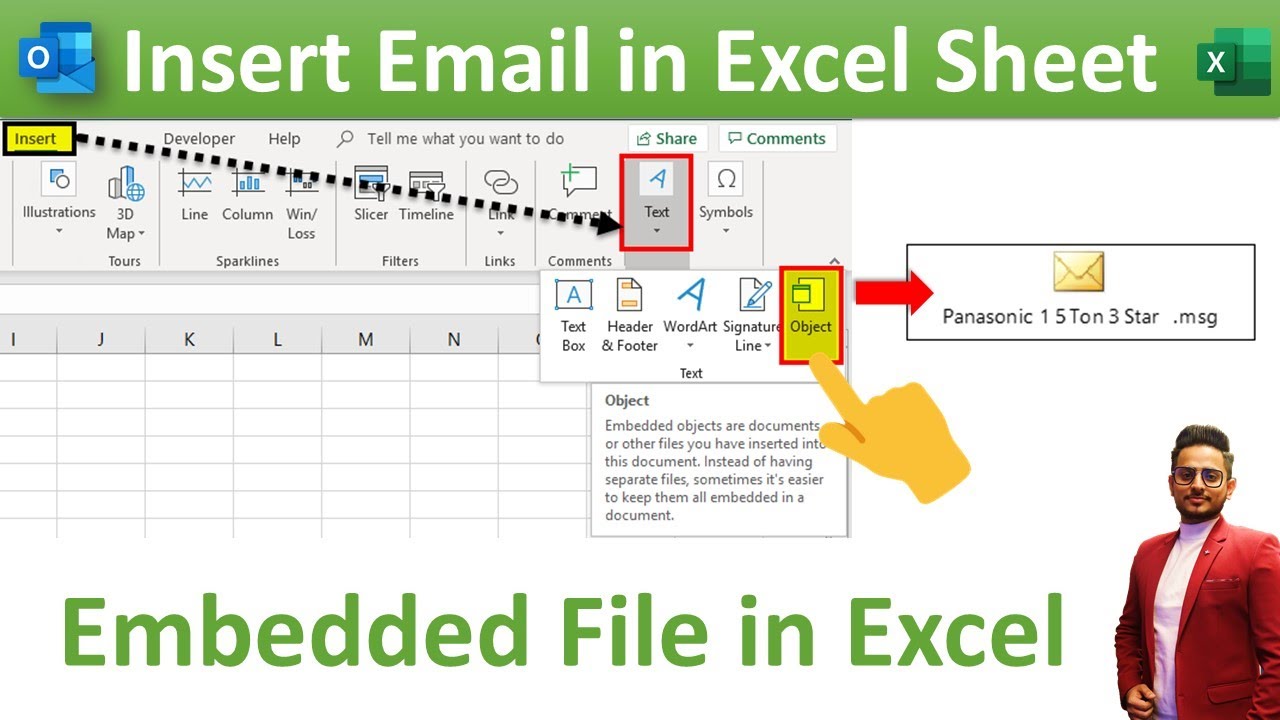
Insert Or Embed An Outlook Email In Excel By Rohit Narang YouTube
https://i.ytimg.com/vi/Ja-OwtVj-Rg/maxresdefault.jpg
The successive ionization energies of some element are I 1 589 5 kJ mol I 2 1145 kJ mol I 3 4900 kJ mol I 4 6500 kJ mol and I 5 8100 kJ mol This pattern of ionization energies An element has successive ionization enthalpy as 940 2080 3090 4140 7030 7870 16000 and 19 500 KJ mole To which group of the periodic table does the element belong t
The successive ionization energies of a certain element are IE1 577 9 kJ mol IE2 1820 kJ mol IE3 2750 kJ mol IE4 11 600 kJ mol and IE s 14 800 kJ mol This pattern of ionization What element in period 3 has the following successive ionization energies IE1 1000 kJ mol IE2 2252 kJ mol IE3 3363 kJ mol IE4 4556 kJ mol 1E5 7004 kJ mol IE6 8496 kJ mol 1E7 27108
More picture related to Attach Email To Microsoft Planner Task
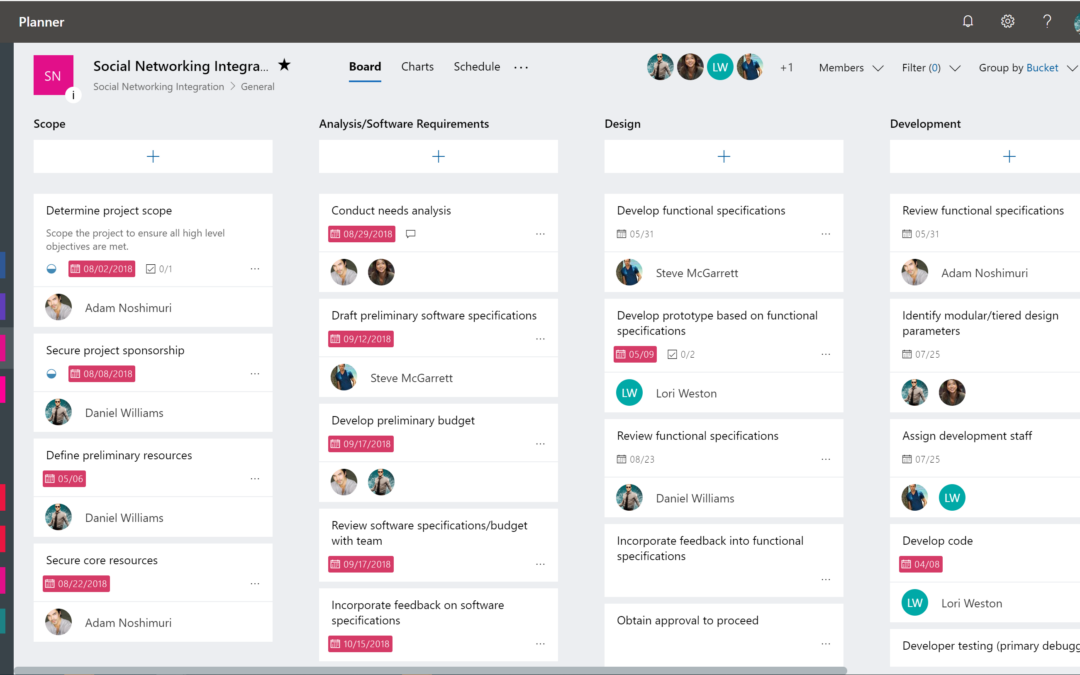
O365 Planner OnePlan
https://projectfortheweb.com/wp-content/uploads/2019/05/O365-Planner-493109_1080x675.png

Premium Vector Schedule App Task Manager UI Template With Project
https://i.pinimg.com/originals/f4/48/94/f44894d809f9d19ef5f98de583e6ff29.png
Microsoft Teams Planner Fulnra
https://1.bp.blogspot.com/-QRcy_x8ooWU/XFRbxJasEPI/AAAAAAAALTw/6W9Jq5sNTu8coojTLA6SyOQ--dEFJkYxQCLcBGAs/s1600/Planner-Colours.png
The successive ionization energies of a certain element are I1 589 5 kJ mol 1 1145 kJ mol Is 4900 kJ mol 14 6500 kJ mol and Is 8100 kJ mol This pattern of ionization energies Based on the successive ionization energies for the following element quot x quot predict the formula that would be formed when quot X quot reacts with chlorine Cl Your solution s ready to go
[desc-10] [desc-11]

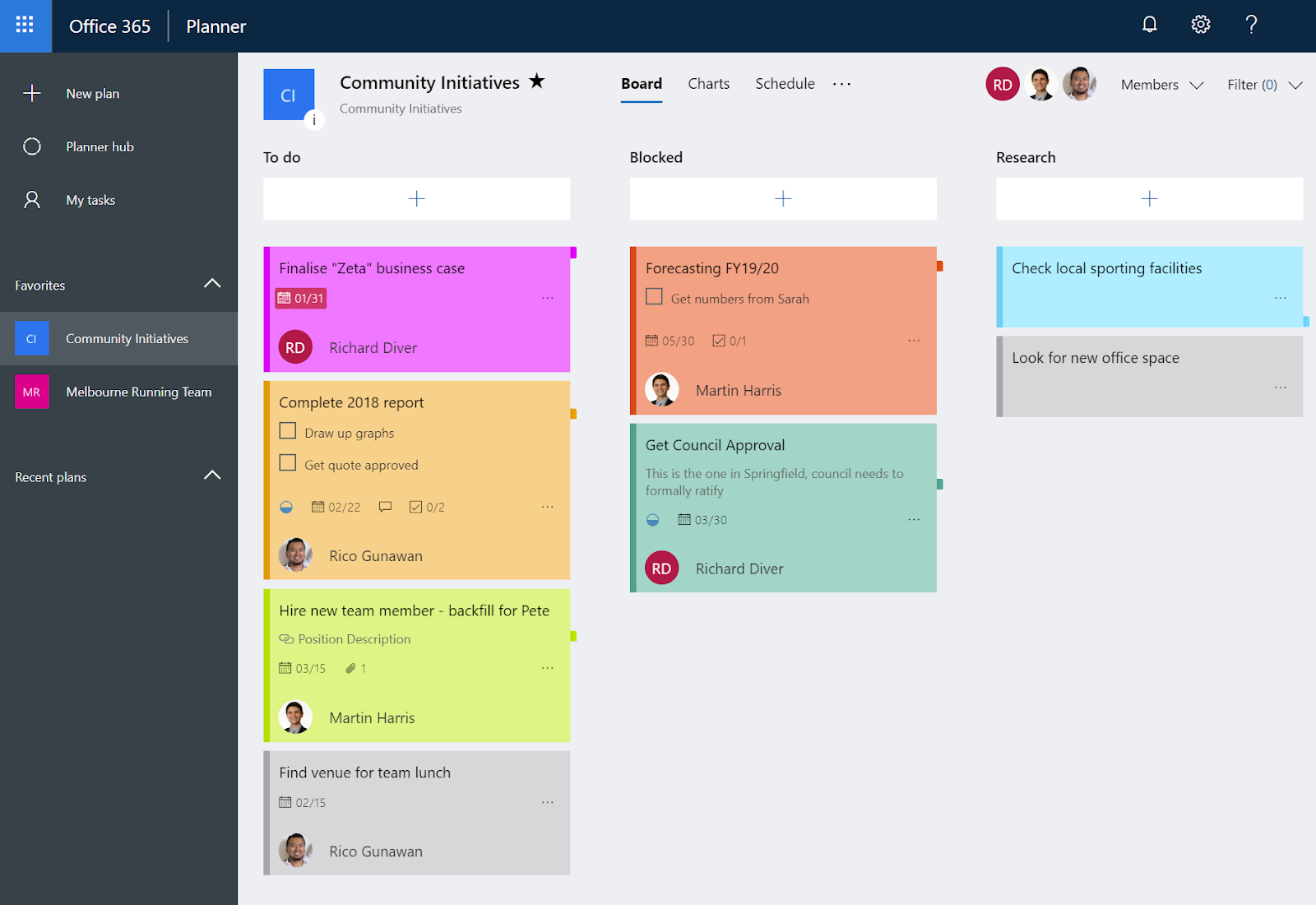
Using Planner In Microsoft Teams The Elm
https://elm.umaryland.edu/announcements/Announcements-Content/57ba877e2dd39f28914157cf1ae7867a.png

Microsoft Teams Planner Ercamping Vrogue co
https://i.ytimg.com/vi/cB2hlnV6QVc/maxresdefault.jpg
Attach Email To Microsoft Planner Task - An element has successive ionization enthalpy as 940 2080 3090 4140 7030 7870 16000 and 19 500 KJ mole To which group of the periodic table does the element belong t