Atomic Structure Isotopes Worksheet Answers ATOMIC STRUCTURE PAST PAPER QUESTIONS Name Mark Element E has an atomic number of 5 a sample of E there are two isotopes One isotope has a mass number of 10 and the Explain in terms of subatomic particles what is meant by the term isotopes
Mar 13 2023 nbsp 0183 32 Understand the basis for atomic theory Understand the structure of atoms isotopes and ions Understand the relationship between the masses of isotopes and the atomic weight of an element Become familiar with the periodic table Mar 12 2022 nbsp 0183 32 Subatomic Structure of Isotopes Isotopes are atoms of the same element that contain the same number of protons and electrons but a different number of neutrons The symbol for an isotope is the chemical symbol or word followed by a dash and then the mass number
Atomic Structure Isotopes Worksheet Answers

Atomic Structure Isotopes Worksheet Answers
https://www.worksheeto.com/postpic/2009/12/mass-and-atomic-number-worksheet_212393.png
16 Molecules And Atoms Worksheet Answer Key Worksheeto
https://www.worksheeto.com/postpic/2009/09/atoms-and-ions-worksheet-answer-key_209032.png
Isotopes And Average Atomic Mass
https://s3.studylib.net/store/data/008720700_1-5ebe2b05fecfe6e076a34d89445b2234.png
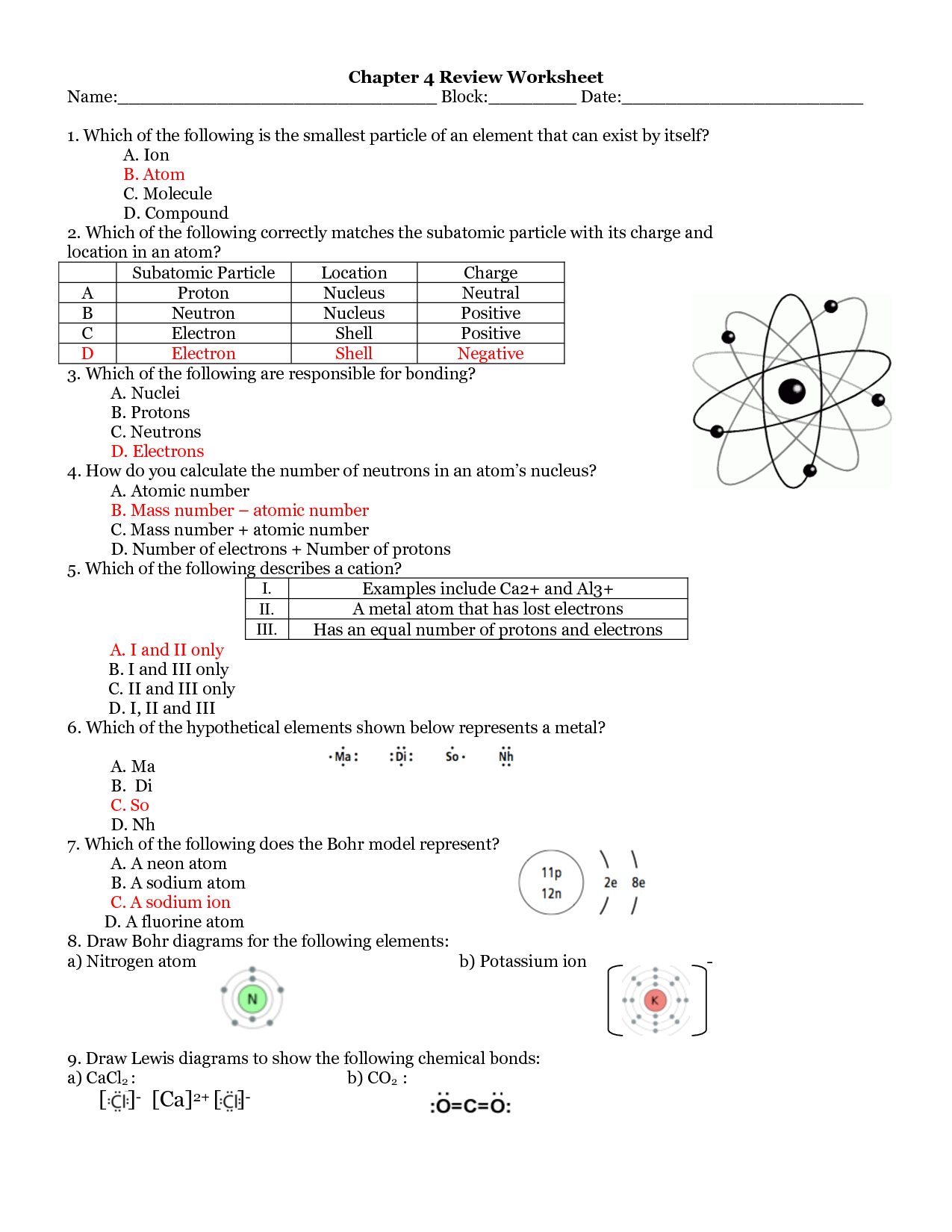
Clearly a confusion between the mass number of an isotope and relative atomic mass see also comments for 21 a Mn was also a common error for the first element presumably by matching the mass number of 54 with the relative atomic mass of Mn 54 9 In a sample of E there are two isotopes One isotope has a mass number of 10 and the other isotope has a mass number of 11 a Explain in terms of subatomic particles what is meant by the term isotopes Isotopes are atoms of the same element that have the same number of protons 1 Iso topes have a different number of neutrons 1 Total
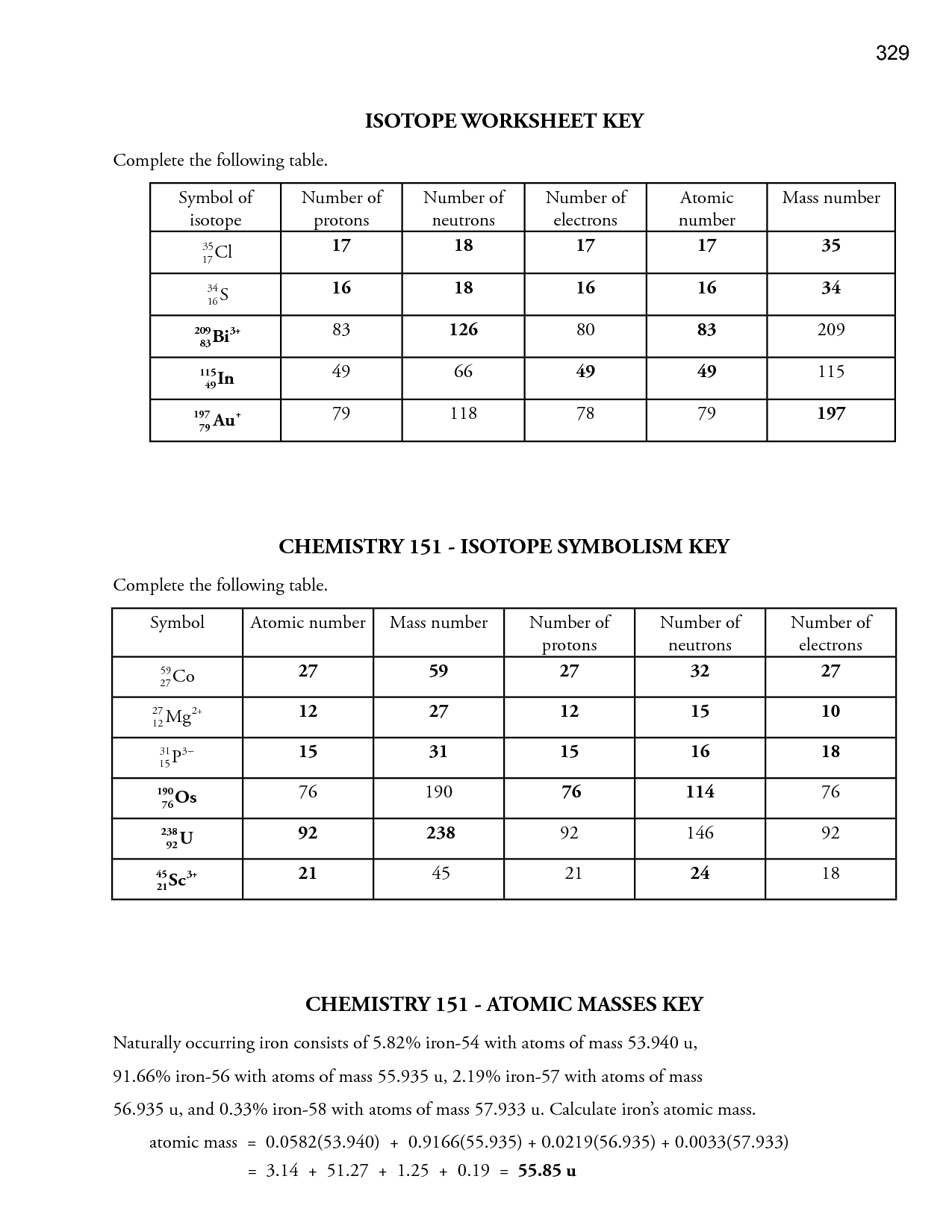
Atoms of the same element with different numbers of neutrons are called isotopes So different isotopes have different mass numbers but the same proton number If we know the number mass number and the atomic number we can calculate the number of neutrons in the atom using Atomic Structure and Isotopes Worksheet with Answers Free download as PDF File pdf or read online for free
More picture related to Atomic Structure Isotopes Worksheet Answers
16 Best Images Of Atomic Structure Worksheet Answer Chart Periodic
http://www.worksheeto.com/postpic/2009/03/isotopes-worksheet-answer-key_212409.png
Calculating Average Atomic Mass Worksheets Answer Key
http://www.unmisravle.com/wp-content/uploads/2018/08/average_atomic_mass_worksheet_answers_with_work_4989965_0.jpg
Worksheet Review Of Atomic Structure And Isotopic Abundance
https://s3.studylib.net/store/data/007723473_2-6119ca98021c1fa006fbe2c0bc2cbc72.png
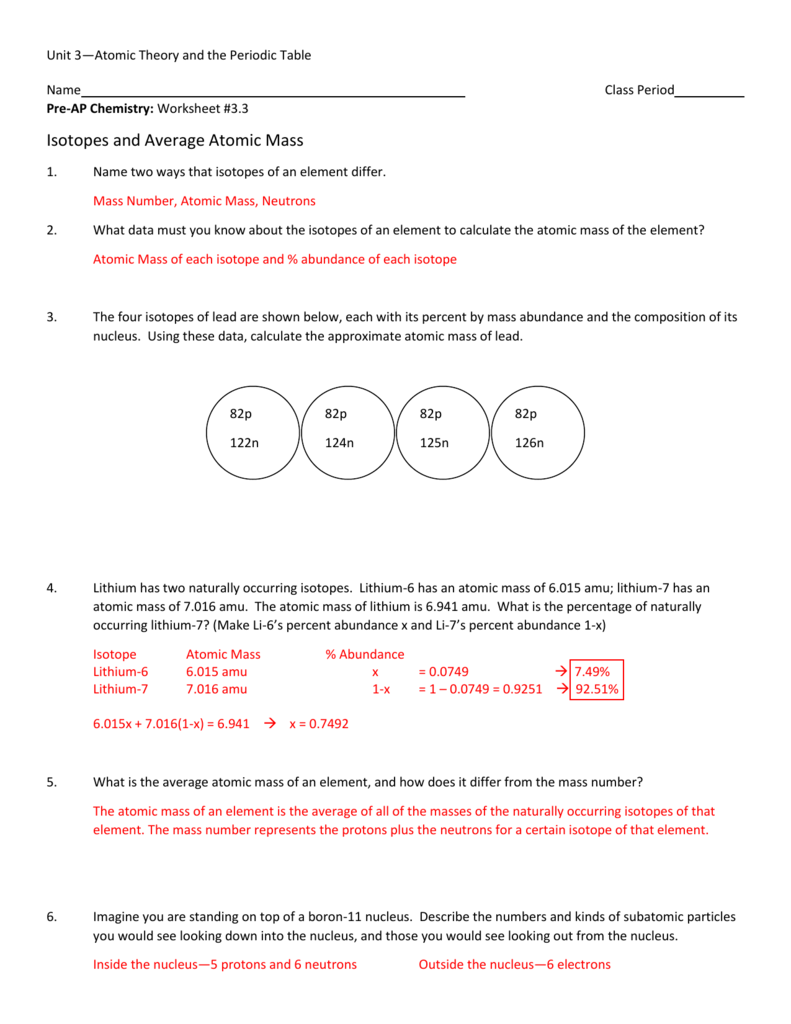
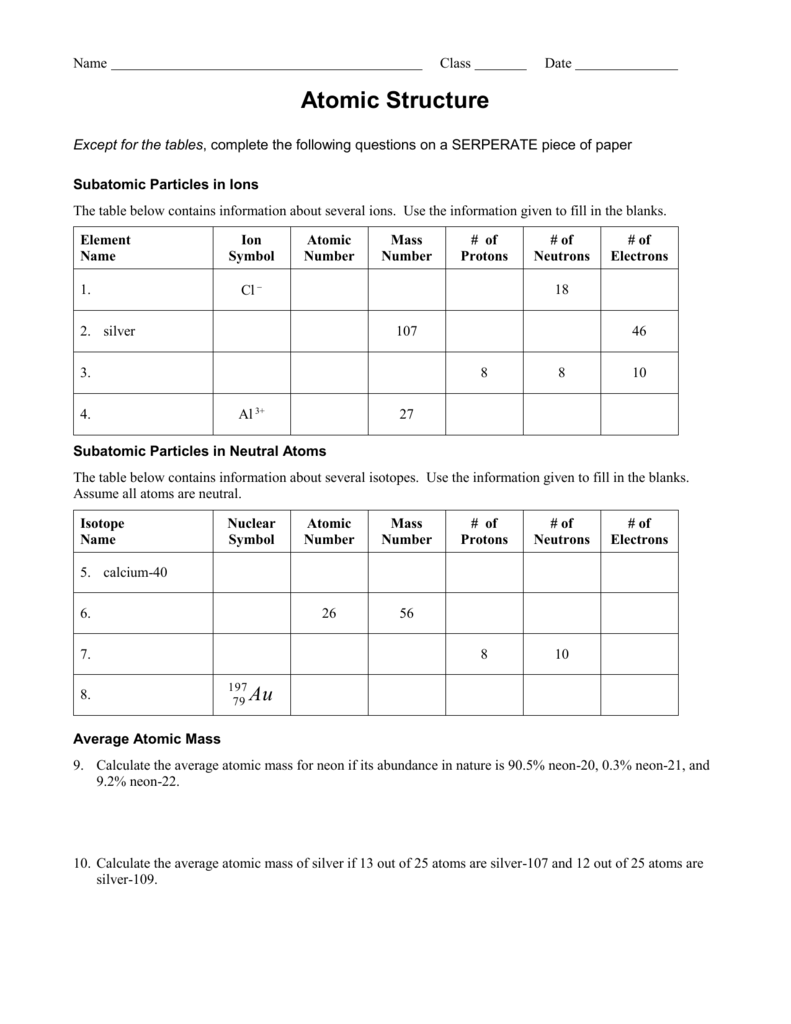
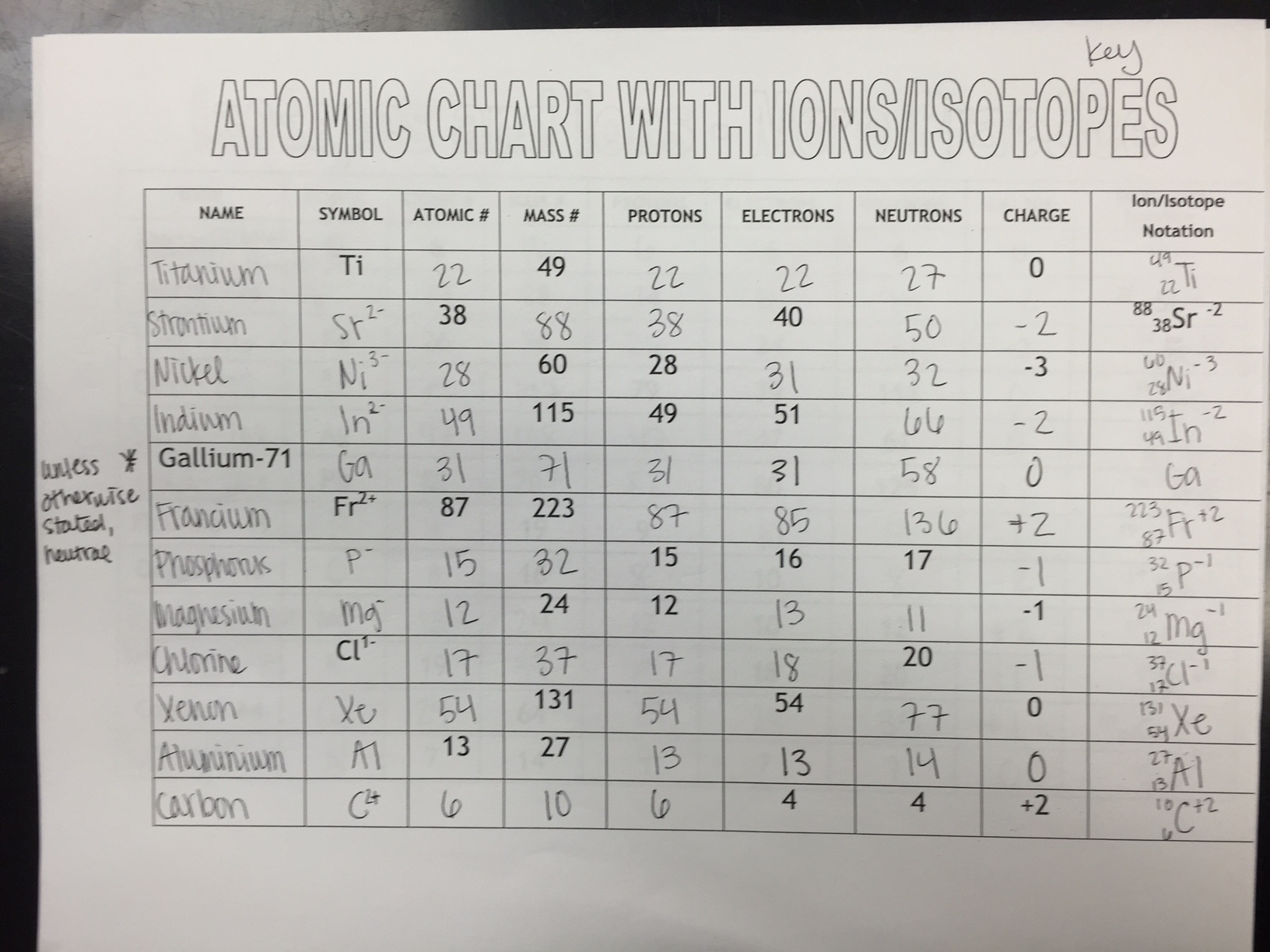
31 The table below gives the atomic mass and the abundance of the two naturally occurring isotopes of chlorine Which numerical setup can be used to calculate the atomic mass of the element chlorine 1 What is an isotope 2 What does the number next to isotopes signify 3 How can you tell isotopes apart For each of the following isotopes write the number of protons neutrons and electrons Fill in the isotope names and any missing information including isotope numbers from the chart Use your periodic table and the information provided
Summary notes revision videos and past exam questions by topic for AQA Chemistry GCSE Topic 1 Atomic Structure and the Periodic Table This worksheet starts with simplified notes on the atom subatomic particles isotopes isotope notation and atomic calculations Then students practice with atomic calculations identifying the atom and writing isotope notation The worksheet ends with questions
Unit 2 Atomic Structure Ms Holl s Physical Science Class
http://hollphysicalscience.weebly.com/uploads/5/8/3/3/58337035/img_1920.jpg

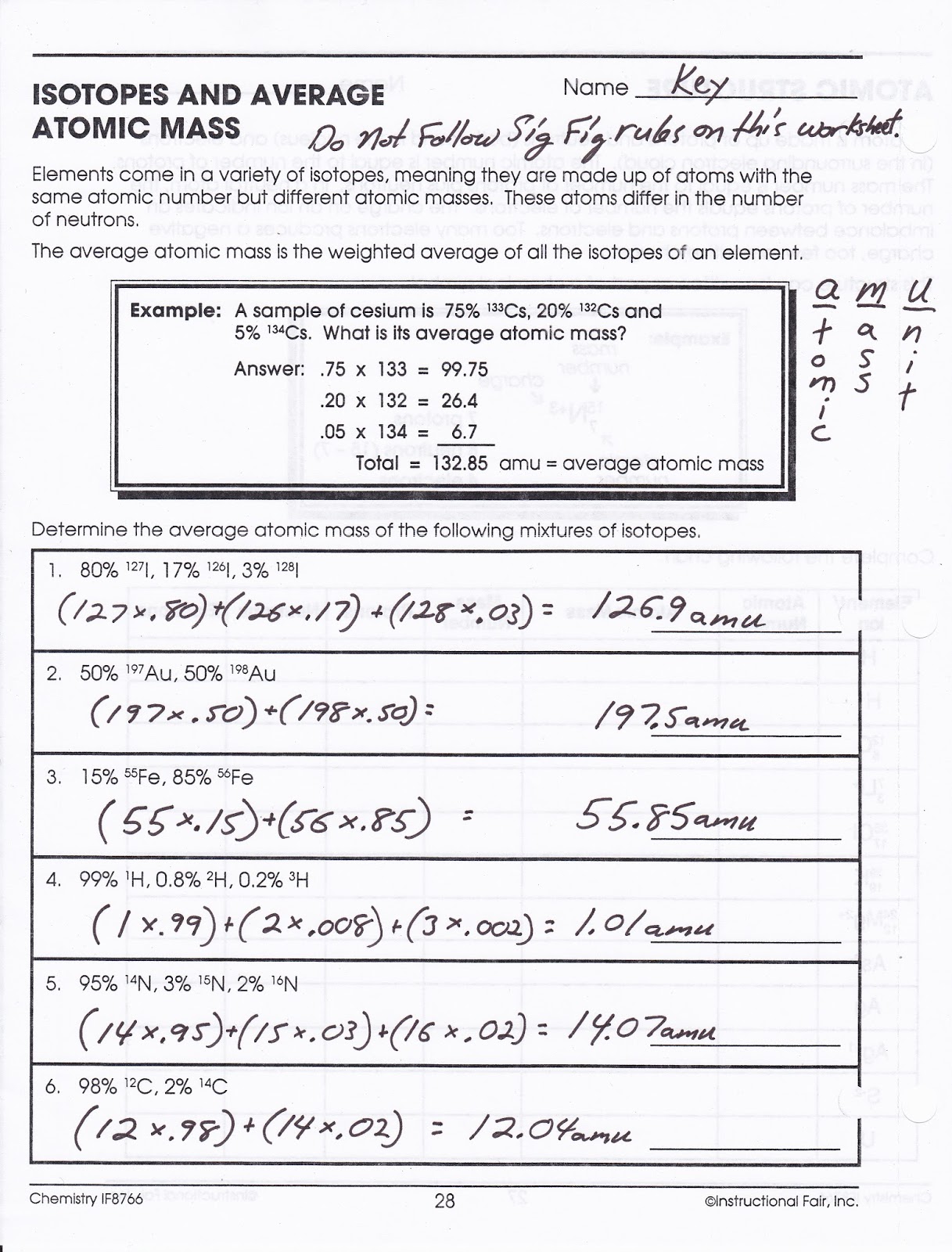
Calculating Atomic Mass Worksheet
https://i.pinimg.com/originals/19/4d/80/194d801cebfaff793da3a3a66ff38ee9.jpg
Atomic Structure Isotopes Worksheet Answers - Atomic Structure and Isotopes Worksheet with Answers Free download as PDF File pdf or read online for free