An Atom With 5 Protons And 6 Neutrons And 5 Electrons Oct 24 2024 nbsp 0183 32 The atom with 5 protons 6 neutrons and 5 electrons is identified as the element Boron specifically its isotope Boron 11 The mass number is 11 calculated from the sum of
Sep 1 2024 nbsp 0183 32 Protons neutrons and electrons of all elements are mentioned in the table below You will get the List Shell diagram of all the elements The atom calculator is a tool for calculating the atomic number and the mass number based on the number of atom components protons neutrons and electrons or vice versa In addition
An Atom With 5 Protons And 6 Neutrons And 5 Electrons
An Atom With 5 Protons And 6 Neutrons And 5 Electrons
https://lh6.googleusercontent.com/proxy/cHmJ4HVruNArIS-g6hNX89WoA7T5gTJJW20eAPyow--172JAhLyOQCyxKdJr3UfUzF_joaggZotDXoiZhW_KT36u-nz_BwYXA2Ja7QWs2wxcbBSZT9x-3qxMsVQkRRJbVs5MypfI8nI84w=s0-d

Protons Neutrons And Electrons Explained The Basics YouTube
https://i.ytimg.com/vi/xzxCwYXz250/maxresdefault.jpg
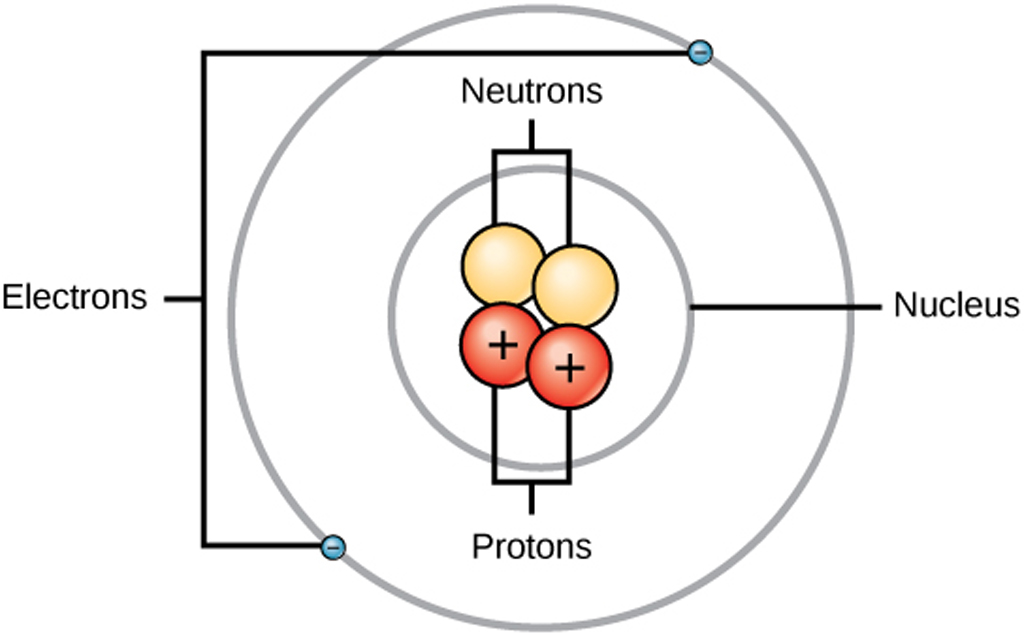
Structure Of An Atom Structure Use Of Electron Proton In Electronics
http://www.electronicsandyou.com/blog/wp-content/uploads/2015/07/atom-structure.jpg
Nov 17 2015 nbsp 0183 32 Mass number protons neutrons The mass number of an element is the sum of the protons and neutrons The number of neutrons for a given element is the only number that May 31 2024 nbsp 0183 32 A boron atom contains 5 protons 5 electrons and usually 6 neutrons It has three electrons in its outer shell making it capable of forming three covalent bonds
May 3 2018 nbsp 0183 32 An atom has 5 protons 5 electrons and 6 neutrons The atomic number number of protons number of electrons 5 The mass number 5 protons 6 neutrons 11 Mar 7 2021 nbsp 0183 32 For example carbon atoms with the usual 6 neutrons have a mass number of 12 6 protons 6 neutrons 12 so they are called carbon 12 What are elements 5 and 6
More picture related to An Atom With 5 Protons And 6 Neutrons And 5 Electrons

Neutron Discovery Difference And More Teachoo Concepts
https://d1avenlh0i1xmr.cloudfront.net/large/9608f5f5-e406-4832-bf83-648f0c2a0609/13.-aluminium-atom-teachoo-01.png

Protons Neutrons And Subatomic Particles Pharmacy Gyan
https://pharmacygyan.com/wp-content/uploads/2021/09/protons.jpeg
How Many Protons Electrons And Neutrons Are In An Atom Presentation
http://www.sliderbase.com/images/referats/1132b/(1).PNG
This element is boron B as it has 5 protons 6 neutrons and normally 5 electrons to maintain a neutral charge The nucleus of an atom has 5 protons and 6 neutrons What would be the a atomic number b mass number c the number of electrons and d the number of valence electrons per atom
The nucleus of an atom has 5 protons and 6 neutrons What would be the a atomic number b mass number c the number of electrons and d the number of valence electrons per atom Oct 14 2019 nbsp 0183 32 In summary an atom with 5 protons and 6 neutrons has 5 electrons and a mass of 11 amu For example consider carbon which has 6 protons and typically 6 neutrons in its most
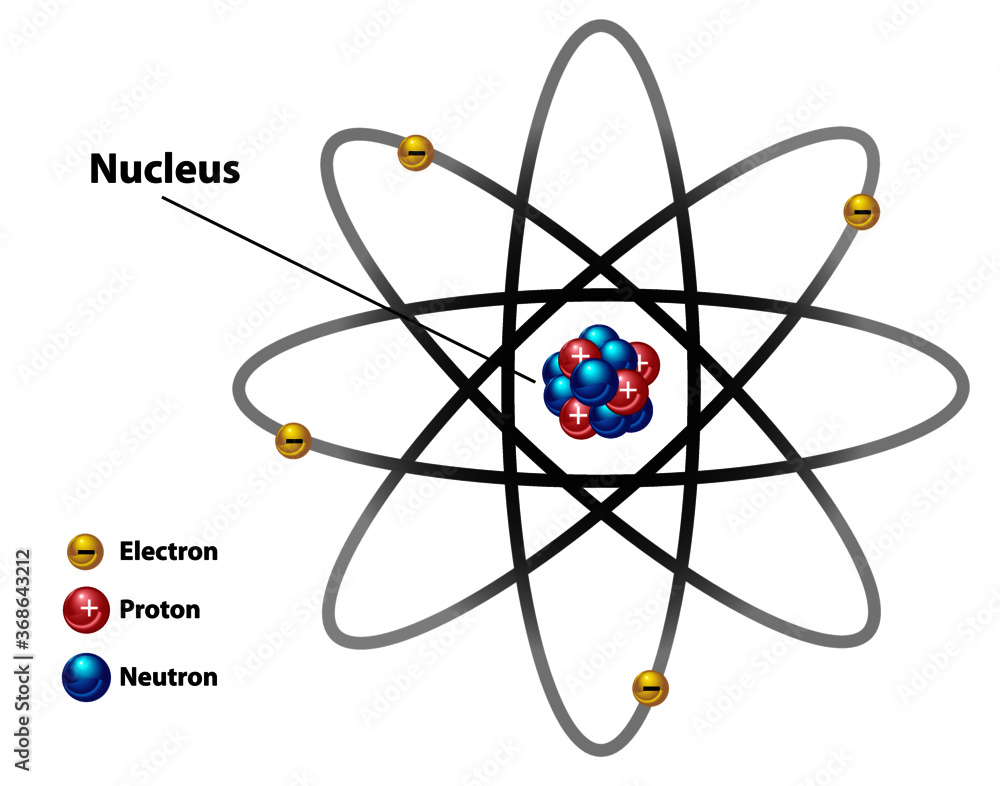
Atomic Nucleus Diagram Labeled With Electron Proton And Neutron
https://as1.ftcdn.net/v2/jpg/03/68/64/32/1000_F_368643212_ovdSJUrD6hM0crl4B03QzcQ55XlyowRS.jpg
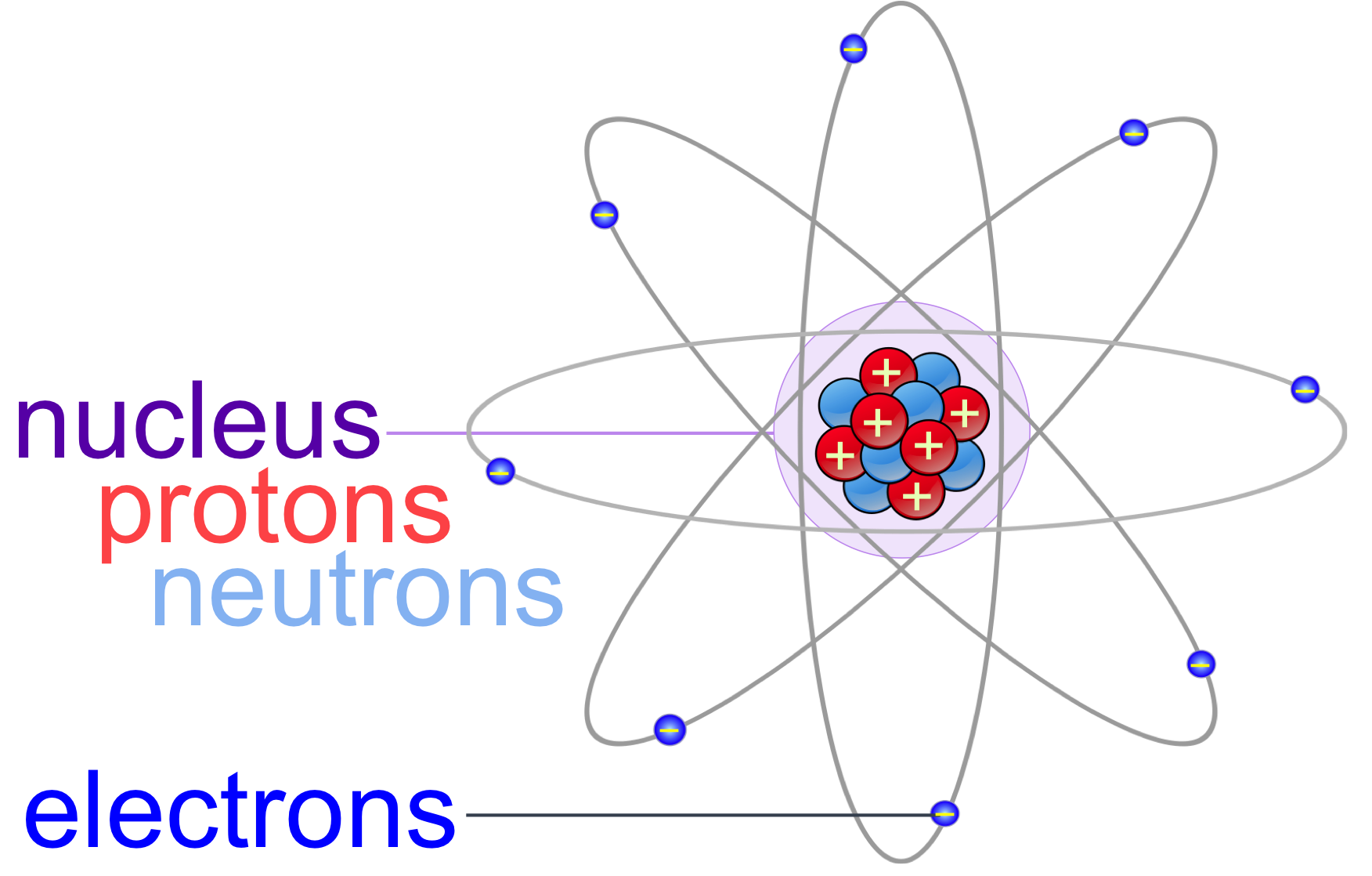
Atoms Molecules E chapter The Biology Primer
https://images.squarespace-cdn.com/content/v1/52668d02e4b0f593739ec2b6/1480796000827-L772BYYF0J5ZJXPVY2LL/ke17ZwdGBToddI8pDm48kD-D-3kKHTrzRk3HArK3pkZ7gQa3H78H3Y0txjaiv_0fDoOvxcdMmMKkDsyUqMSsMWxHk725yiiHCCLfrh8O1z5QPOohDIaIeljMHgDF5CVlOqpeNLcJ80NK65_fV7S1UZ7D58Qd5vUZ457uuIt7W2IHGEb755-i5YiOlab8uRJhG6v6ULRah83RgHXAWD5lbQ/Untitled.png
An Atom With 5 Protons And 6 Neutrons And 5 Electrons - May 30 2024 nbsp 0183 32 To find Atomic Mass add the numbers of protons and neutrons 5 6 11 is the mass number The atomic number is determined by the number of protons in an atom so an
.PNG)