Activation Energy And Reaction Rates Relationship Apply the Arrhenius equation to illustrate the effects of temperature and activation energy on reaction rate and recognize the reaction characteristics represented by the pre exponential factor A Describe how collision frequencies and molecular orientations affect reaction rates
Feb 13 2023 nbsp 0183 32 In the case of a biological reaction when an enzyme a form of catalyst binds to a substrate the activation energy necessary to overcome the barrier is lowered increasing the rate of the reaction for both the forward and reverse reaction In the Arrhenius model of reaction rates activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur 1 The activation energy E a of a reaction is measured in kilojoules per mole kJ mol or kilocalories per mole kcal mol 2
Activation Energy And Reaction Rates Relationship
Activation Energy And Reaction Rates Relationship
https://media.nagwa.com/546193845092/en/thumbnail_l.jpeg

Iconpasob blogg se May 2022
https://lightcat-files.s3.amazonaws.com/problem_images/6b11776b92ca886d-1569863624014.jpg
Factors Affecting The Rate Of A Reaction Temperature W3schools
https://www.w3schools.blog/wp-content/uploads/2019/08/Pasted-into-1-5.png
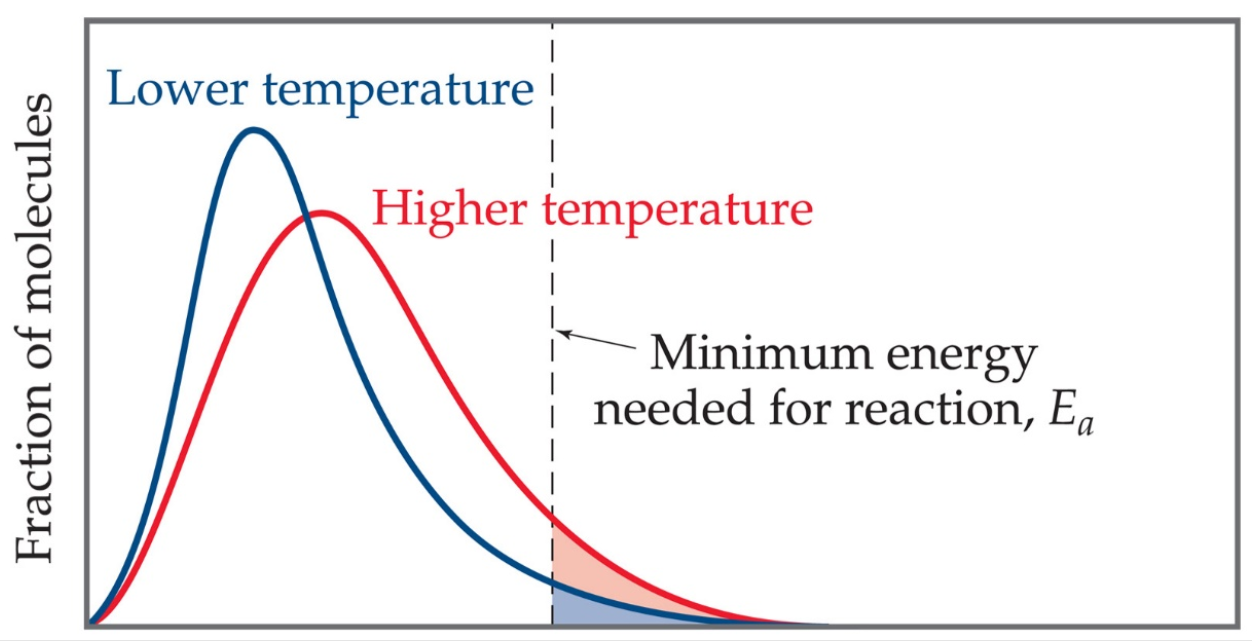
Activation Energy and Reaction Rate The activation energy of a chemical reaction is closely connected to its reaction rate The higher the activation energy the slower the reaction will be For example the rusting of iron is a long process because the activation energy for this reaction is Mar 7 2021 nbsp 0183 32 Activation Energy and Rate of Reaction Activation energy is related to reaction rate The higher the activation energy is the slower the reaction proceeds because fewer reactants have enough energy to overcome the energy barrier at any given time If the activation energy is high enough a reaction won t proceed at all unless energy is
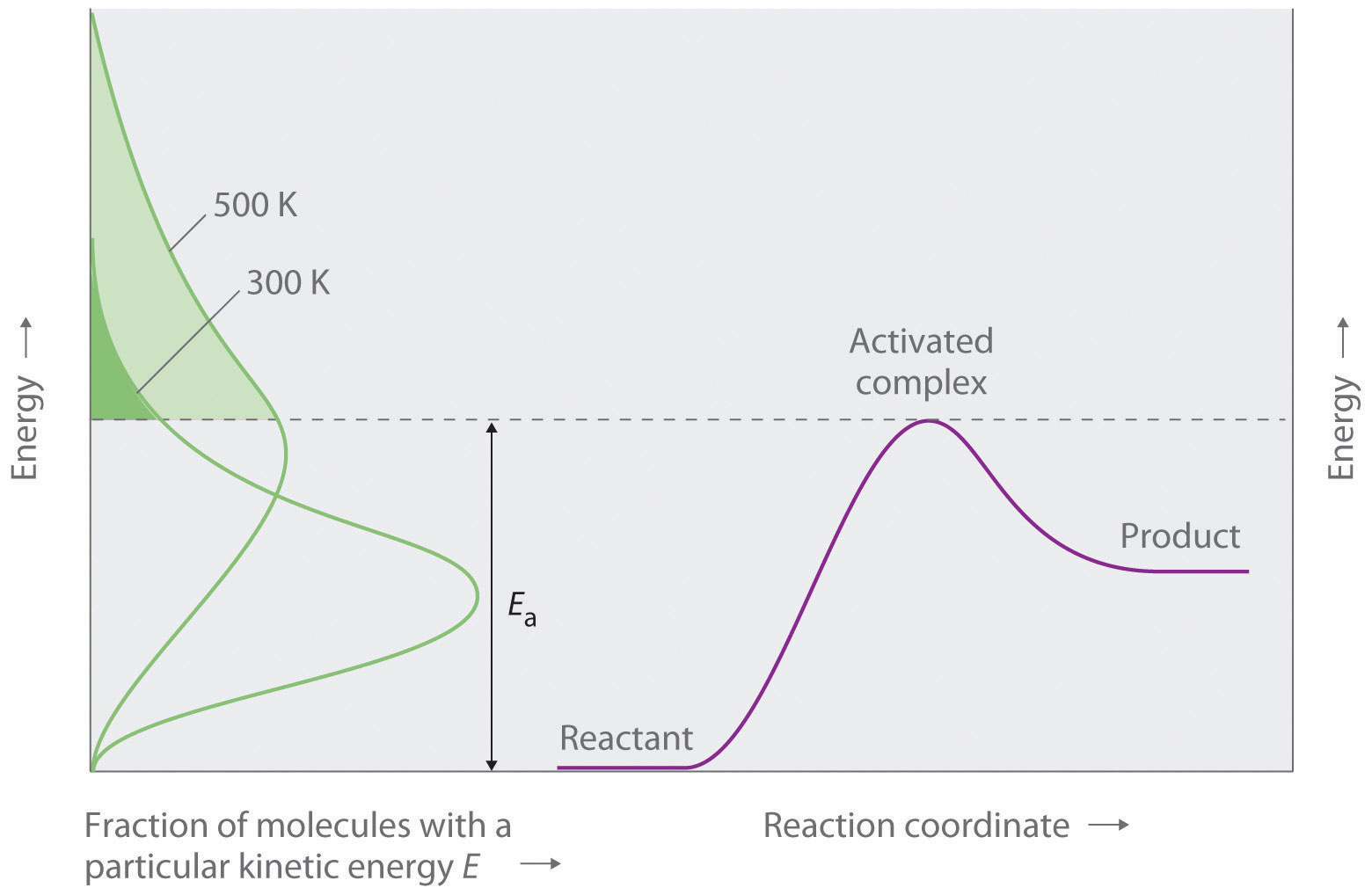
D25 4 Activation Energy Concentration and Reaction Rate The Arrhenius equation enables calculation of the rate constant at a given temperature The rate law enables calculation of the rate given k and concentrations of species that affect the rate Mar 20 2025 nbsp 0183 32 Figure 11 9 1 Reaction profile for an elementary sttep over an activated barrier of height E a If the rate constant for a reaction is measure at two temperatures the activation energy can be determined by taking the ratio This leads to the following expression for the Arrhenius model
More picture related to Activation Energy And Reaction Rates Relationship

Question Video Identifying The Activation Energy Of An Enzyme
https://media.nagwa.com/790171073053/en/thumbnail_l.jpeg
Activation Energy And Temperature Arrhenius Equation Temperature
https://chem.libretexts.org/@api/deki/files/108994/14.24.jpg?revision=1&size=bestfit&width=696&height=454
Collision Theory And Rate Of Reaction Higher Chemistry Unit 1
https://blogs.glowscotland.org.uk/gc/public/hchemunit/uploads/sites/5340/2015/05/Picture3.jpg
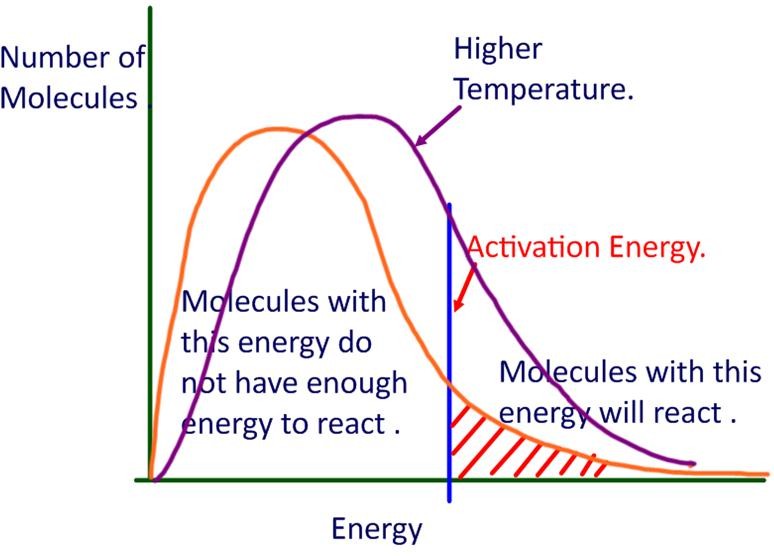
The higher the activation energy the fewer particles in the reaction mixture will have enough energy to react at a given temperature which means the reaction will be slower Conversely if the activation energy is low more particles will have enough energy to react and the reaction will occur more quickly Factors Affecting the Rates of Jun 10 2019 nbsp 0183 32 This empirical relationship between the rate constant and temperature gives rise to the standard approach for determining the activation energy one constructs an Arrhenius plot of ln k T versus 1 T and the slope is then E a k B
Jul 8 2023 nbsp 0183 32 Activation Energy and Rate of Reaction The rate of a reaction relates to the activation energy When the activation energy is high the reaction moves more slowly because fewer reactants have enough energy at any given time to break through the energy barrier The minimum energy necessary to form a product during a collision between reactants is called the activation energy Ea How this energy compares to the kinetic energy provided by colliding reactant molecules is a primary factor affecting the rate of a chemical reaction
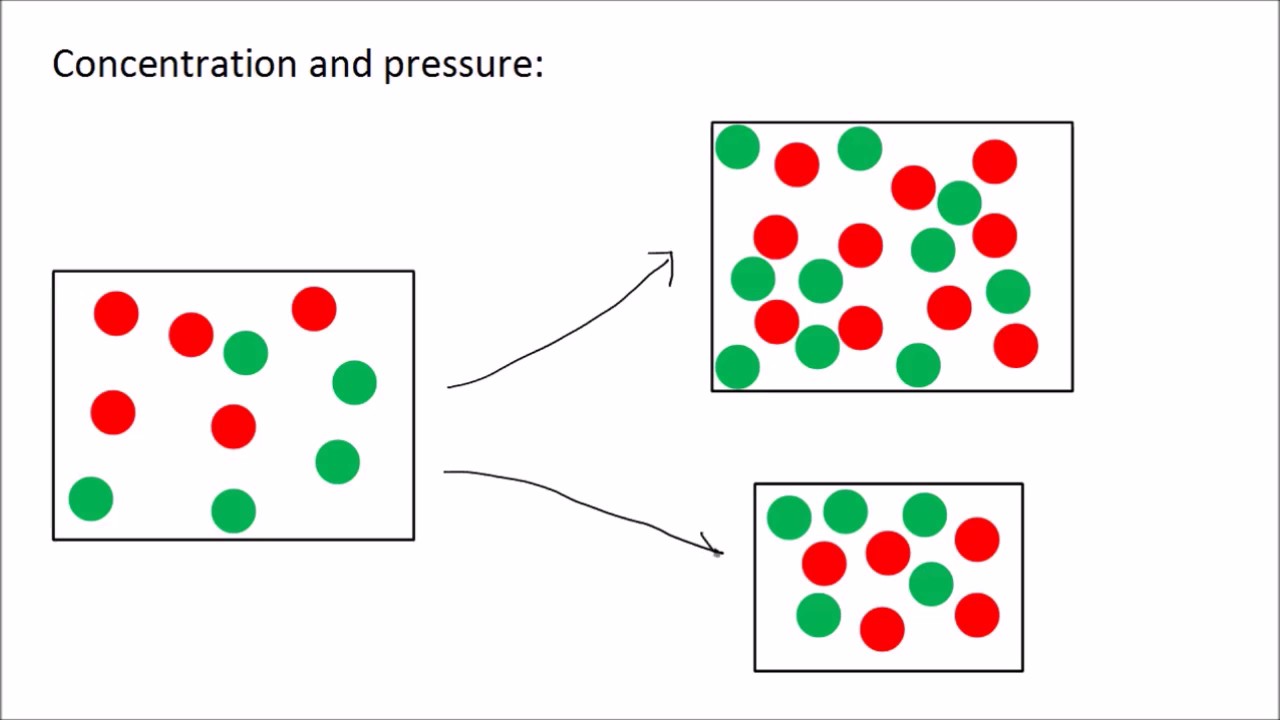
Factors Affecting Rate Of Reaction Collision Theory GCSE Science
https://i.ytimg.com/vi/jd6U5nQcqKc/maxresdefault.jpg
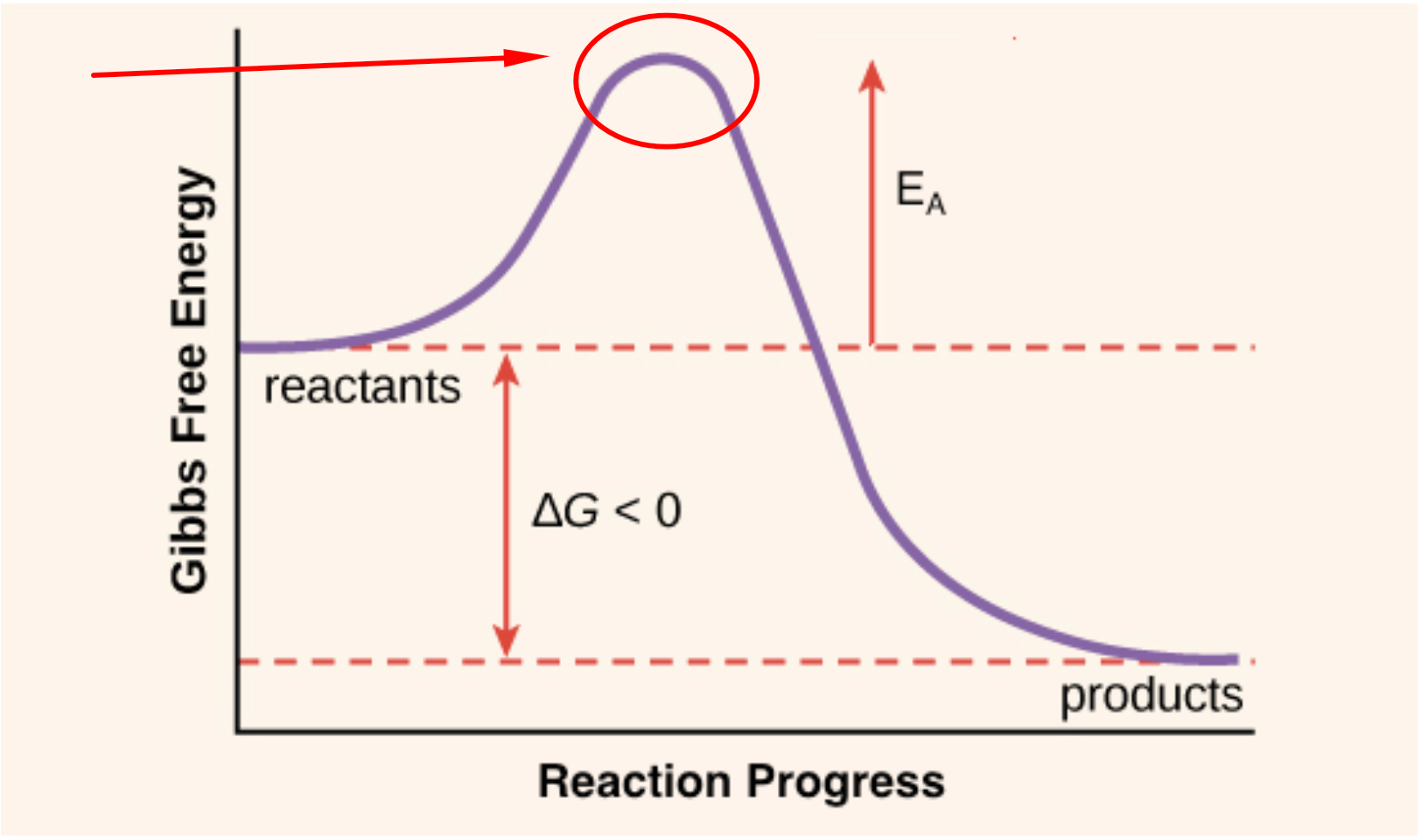
Activation Energy The Secret To Getting Started And Getting Finished
https://www.kosmotime.com/wp-content/uploads/2020/07/image9-1.png
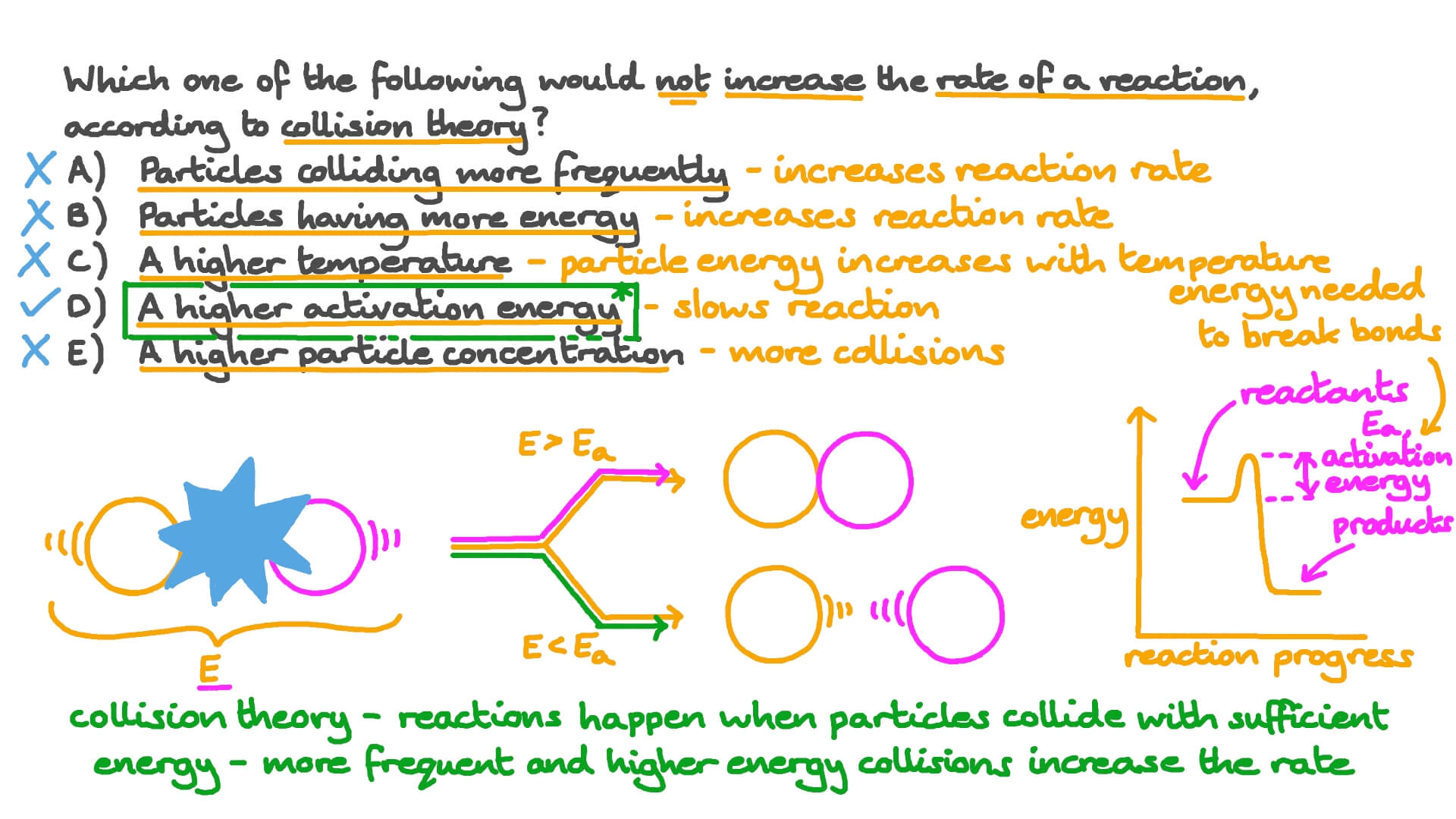
Activation Energy And Reaction Rates Relationship - Activation energy The minimum energy required for a reaction to occur catalysis The increase in the rate of a chemical reaction by lowering its activation energy transition state An intermediate state during a chemical reaction that has a higher energy than the reactants or the products